Sulfur(IV)-Mediated Unsymmetrical Heterocycle Cross-Couplings
Correction(s) for this article
-
Berichtigung: Sulfur(IV)-Mediated Unsymmetrical Heterocycle Cross-Couplings
- Volume 133Issue 11Angewandte Chemie
- pages: 5660-5660
- First Published online: March 1, 2021
Dr. Min Zhou
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorJet Tsien
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorCorresponding Author
Prof. Tian Qin
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorDr. Min Zhou
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorJet Tsien
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorCorresponding Author
Prof. Tian Qin
Department of Biochemistry, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX, 75390-9038 USA
Search for more papers by this authorAbstract
Despite the tremendous utilities of metal-mediated cross-couplings in modern organic chemistry, coupling reactions involving nitrogenous heteroarenes remain a challenging undertaking – coordination of Lewis basic atoms into metal centers often necessitate elevated temperature, high catalyst loading, etc. Herein, we report a sulfur (IV) mediated cross-coupling amendable for the efficient synthesis of heteroaromatic substrates. Addition of heteroaryl nucleophiles to a simple, readily-accessible alkyl sulfinyl (IV) chloride allows formation of a trigonal bipyramidal sulfurane intermediate. Reductive elimination therefrom provides bis-heteroaryl products in a practical and efficient fashion.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| ange201915425-sup-0001-misc_information.pdf10.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Trends in Cross-Coupling: Theory and Applications (Ed.: ), The Royal Society of Chemistry, London, 2015;
- 1b Science of Synthesis: Cross Coupling and Heck-Type Reactions, Workbenh Edition (Eds: ), Thieme, Stuttgart, 2013.
- 2J. F. Hartwig, Organotransition metal chemistry-from bonding to catalyst, University Science Books, Sausalito, 2010, Chapter 8.
- 3For reviews on metal-catalyst cross-couplings, see:
- 3aJ. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359;
- 3bR. F. Heck, Acc. Chem. Res. 1979, 12, 146;
- 3cN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 3dN. Miyaura, Top. Curr. Chem. 2002, 219, 11;
- 3eJ. Terao, N. Kambe, Acc. Chem. Res. 2008, 41, 1545;
- 3fE. I. Negishi, Bull. Chem. Soc. Jpn. 2007, 80, 233;
- 3gT. Hiyama, E. Shirakawa, Top. Curr. Chem. 2002, 219, 61;
- 3hK. Sonogashira, J. Organomet. Chem. 2002, 653, 46;
- 3iJ. K. Stille, Angew. Chem. Int. Ed. Engl. 1986, 25, 508; Angew. Chem. 1986, 98, 504;
- 3jK. Fugami, M. Kosugi, Top. Curr. Chem. 2002, 219, 87.
- 4
- 4aL.-C. Campeau, K. Fagnou, Chem. Soc. Rev. 2007, 36, 1058;
- 4bE. Tyrrell, P. Brookes, Synthesis 2003, 0469;
- 4cM. G. Banwell, T. E. Goodwin, S. Ng, J. A. Smith, D. J. Wong, Eur. J. Org. Chem. 2006, 3043.
- 5
- 5aP. A. Cox, M. Reid, A. G. Leach, A. D. Campbell, E. J. King, G. C. Lloyd-Jones, J. Am. Chem. Soc. 2017, 139, 13156;
- 5bP. A. Cox, A. G. Leach, A. D. Campbell, G. C. Lloyd-Jones, J. Am. Chem. Soc. 2016, 138, 9145.
- 6For reviews, see:
- 6aS. Darses, J.-P. Genet, Chem. Rev. 2008, 108, 288;
- 6bG. A. Molander, N. Ellis, Acc. Chem. Res. 2007, 40, 275; For an example using α-azineboronic ester, see:
- 6cJ. Z. Deng, D. V. Paone, A. T. Ginnetti, H. Kurihara, S. D. Dreher, S. A. Weissman, S. R. Stauffer, C. S. Burgey, Org. Lett. 2009, 11, 345; Selected masked boronic acid coupling methods, see:
- 6dD. M. Knapp, E. P. Gillis, M. D. Burke, J. Am. Chem. Soc. 2009, 131, 6961;
- 6eK. L. Billingsley, S. L. Buchwald, Angew. Chem. Int. Ed. 2008, 47, 4695; Angew. Chem. 2008, 120, 4773; Other stable non-boron electrophiles, see:
- 6fT. Markovic, P. R. D. Murray, B. N. Rocke, A. Shavnya, D. C. Blakemore, M. C. Willis, J. Am. Chem. Soc. 2018, 140, 15916;
- 6gL.-C. Campeau, S. Rousseaux, K. Fagnou, J. Am. Chem. Soc. 2005, 127, 18020.
- 7T. Wang, J. F. Kadow, N. A. Meanwell, K.-S. Yeung, Z. Zhang, Z. Yin, Z. Qui, D. H. Deon, C. A. James, E. H. Ruediger, C. Bachband, US7915283B2, 2011.
- 8
- 8aS. M. Schader, S. P. Colby-Germinario, P. K. Quashie, M. Oliverira, R.-I. Ibanescu, D. Moisi, T. Mespléde, M. A. Waiberg, Antimicrob. Agents Chemother. 2012, 56, 4527;
- 8bRef. [7]. Alternative reported synthesis to compound 6 was shown as below:
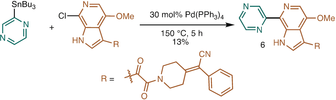
- 9S. Pikul, H. Cheng, A. Cheng, C. D. Huang, A. Ke, L. H. Kuo, A. Thompson, S. Wilder, Org. Process Res. Dev. 2013, 17, 907.
- 10
- 10aB. T. Boyle, M. C. Hilton, A. McNally, J. Am. Chem. Soc. 2019, 141, 15441;
- 10bM. C. Hilton, X. Zhang, B. T. Boyle, J. V. Alegre-Requena, R. S. Paton, A. McNally, Science 2018, 362, 799;
- 10cJ. L. Koniarczyk, J. W. Greenwood, J. V. Alegre-Requena, R. S. Paton, A. McNally, Angew. Chem. Int. Ed. 2019, 58, 14882; Angew. Chem. 2019, 131, 15024.
- 11
- 11aW. A. Sheppard, J. Am. Chem. Soc. 1971, 93, 5597.
- 12
- 12aR. W. LaRochelle, B. M. Trost, J. Am. Chem. Soc. 1971, 93, 6077;
- 12bB. Trost, R. LaRochelle, R. Atkins, J. Am. Chem. Soc. 1969, 91, 2175;
- 12cB. M. Trost, H. C. Arndt, J. Am. Chem. Soc. 1973, 95, 5288.
- 13Y. H. Khim, S. Oae, Bull. Chem. Soc. Jpn. 1969, 42, 1968.
- 14J. Moc, A. Dorigo, K. Morokuma, Chem. Phys. Lett. 1993, 204, 65.
- 15For SOCl2 mediated homocoupling, see: S. Oae, Y. Inubushi, M. Yoshihara, Phosphorus Sulfur Silicon Relat. Elem. 1995, 103, 101.
- 16For selected reviews, see:
- 16aS. Oae, N. Furukawa, Adv. Heterocycl. Chem. 1990, 48, 1;
- 16bS. Oae, Y. Uchida, Acc. Chem. Res. 1991, 24, 202;
- 16cS. Oae, Pure Appl. Chem. 1996, 68, 805; For benzyl pyridine synthesis, see:
- 16dS. Oae, T. Kawai, N. Furukawa, Tetrahedron Lett. 1984, 25, 69;
- 16eS. Oae, T. Kawai, N. Furukawa, F. Iwasaki, J. Chem. Soc. Perkin Trans. 2 1987, 405;
- 16fT. Kawai, Y. Kodera, N. Furukawa, S. Oae, M. Ishida, S. Wakabayashi, Phosphorus Sulfur Relat. Elem. 1987, 34, 139;
- 16gS. Wakabayashi, M. Ishida, T. Takeda, S. Oae, Tetrahedron Lett. 1988, 29, 4441; For phenyl pyridine synthesis, see:
- 16hS. Oae, T. Kawai, N. Furukawa, Phosphorus Sulfur Relat. Elem. 1987, 34, 123; For bipyridine synthesis, see:
- 16iN. Furukawa, T. Shibutani, H. Fujihara, Tetrahedron Lett. 1987, 28, 5845; For allylpyridine and styrylpyridine, see:
- 16jS. Oae, T. Takeda, S. Wakabayashi, Tetrahedron Lett. 1988, 29, 4445; For pyridine homocoupling, see:
- 16kS. Oae, T. Kawai, N. Furukawa, Tetrahedron Lett. 1984, 25, 2549; For ligand exchange through sulfurane intermediate, see:
- 16lN. Furukawa, T. Shibutani, K. Matsumura, H. Fujihara, S. Oae, Tetrahedron Lett. 1986, 27, 3899.
- 17For a review on the application of sulfur chemistry, see:
- 17aD. Kaiser, I. Klose, R. Oost, J. Neuhaus, N. Maulide, Chem. Rev. 2019, 119, 8701; For selected examples of sulfurane ligand coupling reactions in synthesis, see:
- 17bR. W. Baker, D. C. R. Hockless, G. R. Pocock, M. V. Sargent, B. W. Skelton, A. N. Sobolev, E. Twiss (née Stanojevic), A. H. White, J. Chem. Soc. Perkin Trans. 1 1995, 2615;
- 17cR. W. Baker, J. N. H. Reek, B. J. Wallace, Tetrahedron Lett. 1998, 39, 6573;
- 17dR. W. Baker, S. O. Rea, M. V. Sargent, E. M. C. Schenkelaars, T. S. Tjahjandarie, A. Totaro, Tetrahedron 2005, 61, 3733;
- 17eJ. Melzig, C. Rauhut, N. Naredi-Rainer, P. Knochel, Chem. Eur. J. 2011, 17, 5362.
- 18W. M. Dean, M. Šiaučiulis, T. Storr, W. Lewis, R. A. Stockman, Angew. Chem. Int. Ed. 2016, 55, 10013; Angew. Chem. 2016, 128, 10167.
- 19M. Šiaučiulis, A. P. Pulis, D. J. Procter, Angew. Chem. Int. Ed. 2019, 58, 8779; Angew. Chem. 2019, 131, 8871.
- 20D.-L. Chen, Y. Sun, M. Chen, X. Li, L. Zhang, X. Huang, Y. Bai, F. Luo, B. Peng, Org. Lett. 2019, 21, 3986.
- 21D. C. Lenstra, V. Vedovato, E. F. Flegeau, J. Maydom, M. C. Willis, Org. Lett. 2016, 18, 2086.
- 22
- 22aR. M. Beesley, C. K. Ingold, J. F. Thorpe, J. Chem. Soc. Trans. 1915, 107, 1080;
- 22bJ. O'Neill, T. Riesebeck, J. Cornella, Angew. Chem. Int. Ed. 2018, 57, 9103; Angew. Chem. 2018, 130, 9241.
- 23For selected reviews, see:
- 23aT. Mukaiyama, Angew. Chem. Int. Ed. 2004, 43, 5590; Angew. Chem. 2004, 116, 5708;
- 23bJ. Matsuo, J. Synth. Org. Chem. Jpn. 2004, 62, 574.
- 24H. Woolven, C. Gonzalez-Rodríguez, I. Marco, A. L. Thompson, M. C. Willis, Org. Lett. 2011, 13, 4876.
- 25
- 25aJ.-H. Youn, R. Herrmann, Tetrahedron Lett. 1986, 27, 1493;
- 25bF. Izzo, M. Schäfer, R. Stockman, U. Lücking, Chem. Eur. J. 2017, 23, 15189.
- 26For selected reviews, see:
- 26aL. H. S. Smith, S. C. Coote, H. F. Sneddon, D. J. Procter, Angew. Chem. Int. Ed. 2010, 49, 5832; Angew. Chem. 2010, 122, 5968;
- 26bS. K. Bur, A. Padwa, Chem. Rev. 2004, 104, 2401;
- 26cK. Colas, A. Mendoza, Synlett 2018, 29, 1329.
- 27For selected example, see: K. Colas, R. Martín-Montero, A. Mendoza, Angew. Chem. Int. Ed. 2017, 56, 16042; Angew. Chem. 2017, 129, 16258.
- 28Y. Gaoni, J. Org. Chem. 1982, 47, 2564.
- 29A. Krasovskiy, P. Knochel, Angew. Chem. Int. Ed. 2004, 43, 3333; Angew. Chem. 2004, 116, 3396.
- 30A. Krasovskiy, V. Krasovskaya, P. Knochel, Angew. Chem. Int. Ed. 2006, 45, 2958; Angew. Chem. 2006, 118, 3024.
- 31C. Cordovilla, C. Bartolome, J. M. Martínez-Ilarduya, P. Espinet, ACS Catal. 2015, 5, 3040.
- 32
- 32aL. Catherine, R. Alexander, N. Johannes, WO2009/087225 A2;
- 32bThe isolate yield was not reported for this Stille coupling.
- 33J. Wei, H. Liang, C. Ni, R. Sheng, J. Hu, Org. Lett. 2019, 21, 937.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.




