Unraveling the Light-Activated Reaction Mechanism in a Catalytically Competent Key Intermediate of a Multifunctional Molecular Catalyst for Artificial Photosynthesis
Abstract
Understanding photodriven multielectron reaction pathways requires the identification and spectroscopic characterization of intermediates and their excited-state dynamics, which is very challenging due to their short lifetimes. To the best of our knowledge, this manuscript reports for the first time on in situ spectroelectrochemistry as an alternative approach to study the excited-state properties of reactive intermediates of photocatalytic cycles. UV/Vis, resonance-Raman, and transient-absorption spectroscopy have been employed to characterize the catalytically competent intermediate [(tbbpy)2RuII(tpphz)RhICp*] of [(tbbpy)2Ru(tpphz)Rh(Cp*)Cl]Cl(PF6)2 (Ru(tpphz)RhCp*), a photocatalyst for the hydrogenation of nicotinamide (NAD-analogue) and proton reduction, generated by electrochemical and chemical reduction. Electronic transitions shifting electron density from the activated catalytic center to the bridging tpphz ligand significantly reduce the catalytic activity upon visible-light irradiation.
Introduction
Solar-driven water splitting to release molecular hydrogen as a carbon-neutral energy source is an attractive solution to satisfy the rising global energy demand.1 Despite considerable progress, artificial photosynthesis does not reach its full potential in sustainably converting solar energy into chemical energy. Attempts to develop visible-light-driven hydrogen-evolution catalysts generally include a photosensitizer for light harvesting, an inter- or intramolecular electron relay to achieve charge separation, and a catalytic center for hydrogen generation. Many approaches for heterogeneous and homogeneous systems have been established and spectroscopically studied to elucidate the reaction mechanism and to explore the factors determining the catalytic activity.2 However, intramolecular photocatalytic reactions often involve complex multi-step reactions with short-lived and highly reactive intermediates. Since water splitting is an inherent multielectron process, elucidation of the properties of the redox-activated intermediates is of paramount importance to understanding the overall catalytic activity. The identification of those intermediates and their excited-state processes within the electron-transfer cascades are of fundamental importance for an understanding of the catalytic mechanism and the identification of competing deactivation pathways. Therefore, detailed knowledge with respect to the structure, photophysical properties, and photoinduced dynamics of short-lived photoexcited intermediates is of utmost importance to develop highly active and stable photocatalysts for hydrogen production and other uses.
Therefore, the presented work investigates these intermediates which result from photoexcitation and subsequent electron transfer by spectroelectrochemical methods (UV/Vis absorption and resonance-Raman (rR) spectroscopy) in combination with quantum-chemical simulations.3 rR spectroscopy is specifically suitable to identify the initially photoexcited state because only those vibrations are enhanced that are connected to structural changes coupled to the electronic transition.4 Time-resolved transient absorption (TA) spectroelectrochemistry (SEC) enables the investigation of the photoinduced electron transfer in intermediates of the catalytic cycles on femto-to-nanosecond timescales.
Here, we combine rR- and TA-SEC with the photocatalytic characterization of a key intermediate in the photocatalytic cycle of [(tbbpy)2Ru(tpphz)Rh(Cp*)Cl]Cl(PF6)2 (Ru(tpphz)RhCp*) (Figure 1 A, structure on the left).5 The catalyst features a RuII chromophore, a tetrapyridophenazine (tpphz) bridging ligand, and a RhIII(Cp*)Cl catalytic center. In the presence of sacrificial electron donors, it photocatalytically reduces nicotine amides, which are biologically usable NAD-like (nicotinamide adenine dinucleotide) cofactors (Figure 1 A).6 The preorganized structure of the chromophore and the catalyst prevents the formation of bioinactive NAD(P) dimers.6, 7 Additionally, catalytic hydrogen evolution was observed for more than six times longer (650 hours) than for structurally similar tpphz-bridged photocatalysts.2c, 8 However, the use of Ru(tpphz)RhCp* as a photocatalyst for hydrogen production shows a catalytic activity much lower than for NAD+ reduction. Thus, the study presented here focuses on the mechanism of the light-driven reduction of NAD-like cofactors. The reductively fully activated intermediate in the respective photocatalytic cycle containing two reduction equivalents, that is, [(tbbpy)2RuII(tpphz)RhICp*] (Figure 1, center), is likely formed in presence of an excess of a sacrificial electron donor upon irradiation with visible light. Here we exploit electrochemical reduction and chemical reduction with CoCp2 to form this species. It should be noted that finding a suitable reductant to access the catalytically competent intermediate presents a delicate task, because the respective redox potentials have to be balanced to selectively reduce the Rh ion only, a task, however, easily accomplished by electrochemical reduction. The catalytic competence of the fully reduced Ru(tpphz)RhCp* towards the formation of reduced nicotine amides is shown (Figure 1).6 To the best of our knowledge, we present, for the first time, the results of early-time photodynamics of electrochemically generated intermediates of a fully competent photocatalyst, that is, the doubly reduced Ru(tpphz)RhCp*, under non-catalytic conditions to gain important mechanistic insights into electron-transfer cascades occurring during the catalytic cycle. These joint catalytic–spectroscopic mechanistic insights provide a detailed picture of structure–function–activity correlations in this class of photocatalysts for artificial photosynthesis.
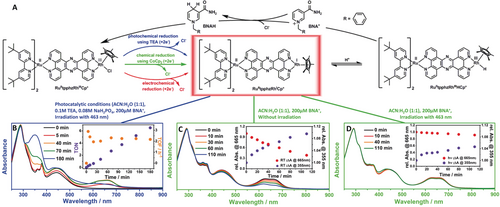
A) Molecular structure of the hetero-binuclear photocatalyst (left). Cleavage of the chloro ligand is observed during the 2 e− reduction, resulting in a RhI intermediate RuII(tpphz)RhICp* (framed in red). The general mechanism of the light-driven catalytic hydrogenation of N-benzylnicotinamide (BNA+ to BNAH) is displayed, including various possibilities to generate the fully charged RuII(tpphz)RhICp* (blue: photocatalytic conditions; green: chemical reduction with CoCp2; red: electrochemical reduction). B) UV/Vis-spectroscopic monitoring of the light-driven (λexc=463 nm) catalytic hydrogenation of a nicotinamide using 20 μm Ru(tpphz)RhCp* in ACN:H2O=1:1 with 0.1 m TEA and 0.08 m NaH2PO4. Inset: Calculated catalysis parameters TON and TOF based on the spectroscopic changes at 355 nm induced by the formation of BNAH. C), D) UV/Vis-spectroscopic changes during addition of 200 μm of the subtrate BNA+ to a solution containing chemically (by CoCp2) reduced Ru(tpphz)RhCp* during irradiation with a 463-nm LED stick. Insets: Relative absorbance changes at 665 nm and 355 nm with time.
Results and Discussion
Figure 1 schematically indicates key reaction steps underlying the light-driven multielectron proton-coupled hydrogenation of N-benzylnicotinamide (BNA+) by Ru(tpphz)RhCp*. To unravel mechanistic details of the reaction, specifically the photoinduced reactivity of the key intermediate (shown in the red box of Figure 1), the photocatalytic reaction was first monitored by UV/Vis spectroscopy (Figure 1 B). In a second step, the catalytically active species (Figure 1 A, center) was generated by chemical reduction with CoCp2 and tested for its reactivity (Figure 1 C,D and Figure S18 in the Supporting Information). Here, irradiation of the chemically doubly reduced Ru(tpphz)RhCp* in presence of BNA+ significantly hampered the catalytic turnover, as indicated by comparing the much stronger absorbance increase at 355 nm (ascribed to the formation of the reduced product BNAH) in the dark compared to the same sample under continuous irradiation with visible light. Finally, the intermediate was prepared in an electrochemical approach and characterized by UV/Vis, rR-SEC, TA-SEC, and quantum-chemical simulations. The electrochemical characterization reveals that the first and second reduction in Ru(tpphz)RhCp* is located at the Rh ion: The irreversible reduction at −0.6 V vs. a silver pseudo-reference electrode is due to a two-electron process in the RhIII/I pair (Figure 2 B, inset).9 This process can be explained by two possible pathways: Either a one-electron reduction is followed by fast disproportion of the RhII intermediate,9b or potential inversion of the redox potential of the RhII/RhI and RhIII/RhII pairs allows for an ECE (electrochemical reaction, chemical reaction, electrochemical reaction) process at the applied potential.10 The reduction induces a loss of the chloro ligand, altering the original piano-stool geometry of the RhIII(Cp*)Cl center in Ru(tpphz)RhCp*, that is, the plane of the cyclopentadienyl ligand is now oriented perpendicular to the tpphz ligand (Figure 1 A, center).11 In analogy with previous studies, the reduction at −0.9 V is assigned to the first reduction of the tpphz ligand (Figure 2 B).5 Ru(tpphz)RhCp* exhibits similar absorption properties as the structurally related compounds Ru(tpphz)PdCl2 and Ru(tpphz)PtX2 (X=Cl, I).3b, 12 The different catalytic centers do not affect the steady-state absorption properties significantly.5, 13 The band at 440 nm is due to metal-to-ligand charge-transfer (MLCT) transitions from the RuII center to both the tpphz and the tbbpy ligands (Figure 2 B).12 Bands between 350 and 400 nm stem from tpphz-centered π–π* transitions, whereas the sharp band peaking at 286 nm is due to intra-ligand π–π* transitions located at the Cp* and the tbbpy moieties. Upon chemical reduction of Ru(tpphz)RhCp* by CoCp2, a broad absorption at 650 nm arises, while the spectral changes in the other spectral regions remain minute (Figure 2 A). The same was observed for the photochemical reduction of Ru(tpphz)RhCp* in presence of TEA (Figure S17). These reduction-induced changes are also apparent upon electrochemical reduction (Figure 2 B), which can be performed at specific potentials enabling a stepwise reduction of Ru(tpphz)RhCp*. A similar absorption feature was previously observed for singly reduced Ru(tpphz)PtCl2 and assigned to an intra-ligand π–π* transition of the reduced tpphz ligand by rR-SEC and TD-DFT simulations (red line, Figure 2 C).3b This startling coincidence can be rationalized considering that photoexcitation of the reduced Ru(tpphz)RhCp* may occur at the RhI center, that is, a MLCT from the RhI to the tpphz ligand occurs. Such a transition is observed in the same range as intra-ligand π–π* transitions of the reduced tpphz ligand, as evident from studies on the model complex (phen)RhICp*.14 Further evidence is provided by means of quantum-chemical calculations, predicting bright MLCT transitions into the S7 state at 606 nm (Table S4 and Figure S8) and the T11 state at 652 nm (Table S5 and Figure S10) for the doubly reduced Ru(tpphz)RhCp* within singlet and triplet multiplicity, respectively. UV/Vis-SEC during the third reduction of Ru(tpphz)RhCp* reveals a strong absorption increase between 500 and 800 nm, which is related to 2MLCT transitions from RhI to the tbbpy ligands (D23 and D24) as well as from RhI to the tpphz ligand (D25), and to the intra-ligand π–π* transition (D28) of the now reduced tpphz ligand (blue line, Figure 2 B and C, see Supporting Information for details).
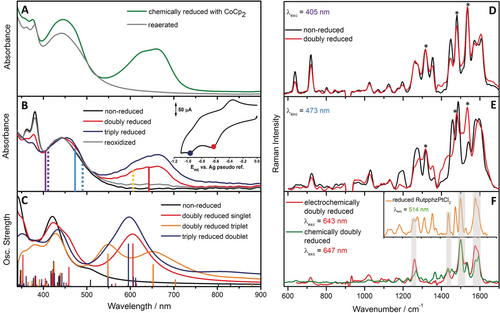
A) UV/Vis spectrum of the chemically reduced Ru(tpphz)RhCp* (green trace) using CoCp2 as a reducing agent and the spectrum recorded after reaeration (grey trace) of the solution which regenerates the parent complex. B) Experimental in situ and C) calculated UV/Vis spectroelectrochemical results of the reduction states of Ru(tpphz)RhCp*. Inset in (B): Cyclovoltammetry of Ru(tpphz)RhCp* in ACN containing 0.1 m TBABF4 electrolyte. The applied potentials for the acquisition of the UV/Vis spectra are marked in red and blue (scan rate 100 mV s−1, glassy carbon working electrode, Pt counter electrode, and Ag/AgCl pseudo-reference electrode). rR excitation and transient-absorption pump wavelengths are displayed as continuous and dashed vertical lines in the spectra, respectively. D)–F) Experimental rR spectra of non-reduced (black), electrochemically doubly reduced (red) and chemically reduced (green) Ru(tpphz)RhCp*, excited at 405 (D), 473 (E), as well as 643 and 647 nm (F). Modes assigned to the tbbpy ligands are marked with an asterisk. Inset: rR spectrum of reduced Ru(tpphz)PtCl2, excited at 514 nm, shown for comparison. Gray-shaded Raman bands are assigned to tpphz vibrations.
To reveal structural changes of Ru(tpphz)RhCp* upon reduction, rR-SEC was performed at 405, 473, and 643 nm (Figure 2 D–F).3b, 3c, 4, 12, 15 At 405 and 473 nm, MLCT transitions are probed which involve both the tbbpy and the tpphz ligands around the RuII center.4, 16 In line with previous studies on closely related complexes, the contribution of the tpphz ligand to the rR spectra is higher upon 473-nm excitation as compared to excitation at 405 nm (Figure 2 D and E).12, 13, 17
RhIII/RhI reduction causes only minor changes in the rR spectra of Ru(tpphz)RhCp* excited at 405 nm (Figure 2 D): The tbbpy-assigned bands at 1320, 1480, and 1540 cm−1 roughly keep their intensity upon reduction, while tpphz-associated modes decrease notably. Also, the rR spectra recorded at 473 nm (Figure 2 E) show an intensity increase of tbbpy-related bands, while the intensity of tpphz-associated bands diminishes. Hence, photoexcitation of the reduced Ru(tpphz)RhCp* at 405 and 473 nm leads to a tbbpy-MLCT being populated. Reduction of the Rh-center appears to impede a MLCT transition to the tpphz ligand. This finding agrees with the quantum-chemical calculations and a previous report18 showing the excess charge at the Rh center to be partially delocalized on the phenanthroline fragment of the tpphz ligand (see Figure S15). This charge distribution apparently inhibits (additional) charge transfer from the RuII towards the tpphz ligand (Figure S8).
rR-SEC at 643 nm samples the reduction-induced absorption band at 650 nm, which is characteristic for the doubly reduced Ru(tpphz)RhCp* (Figure 2 B). The rR spectrum at 643 nm is dominated by features which are neither visible upon excitation at 405 nor 473 nm (Figure 2 F). Particularly, no bands associated with the tbbpy ligands are observed, pointing to the fact that the reduction-induced transition in the red part of the UV/Vis absorption spectrum does not involve excitation of the RuII center and the tbbpy ligands. Excitation of a RhI→Cp* transition is unlikely due to the large electron density of the ligand.9c, 14 However, the 643-nm rR-SEC spectrum compares to the spectrum of reduced Ru(tpphz)PtCl2 (Figure 2 F, inset). Ru(tpphz)PtCl2 is structurally similar to Ru(tpphz)RhCp*, but the first reduction is localized on the tpphz ligand instead of the catalytic metal center.3b When comparing the 643-nm rR spectrum of the reduced Ru(tpphz)RhCp* with the respective spectrum of the reduced RuII(tpphz⋅−)PtIICl2 excited at 514 nm, it becomes evident that the bands observed for the reduced Ru(tpphz)RhCp* are associated with the tpphz ligand. Hence, either a RhI→tpphz MLCT or a tpphz intra-ligand (IL) is excited at 643 nm. The rR-SEC studies do not allow for discrimination between these two scenarios. TD-DFT simulations of the doubly reduced Ru(tpphz)RhCp*, both in singlet and triplet multiplicity, show bright MLCT transitions from the RhI center to the tpphz ligand, partially mixed with a local excitation of the tpphz ligand, see S7 (606 nm) and T11 (at 652 nm) in Figure 2 C. Furthermore, a bright IL transition centered on the tpphz ligand (T21), typical for the reduced tpphz, is predicted by TD-DFT at 548 nm for the doubly reduced triplet species.
Thus, rR-SEC shows that reduction of the RhIII center in Ru(tpphz)RhCp* alters the electronic transitions available at the RuII photocenter, that is, the additional charge density upon RhIII/RhI reduction becomes partially delocalized over the tpphz ligand and prohibits further charge-density shift on the bridging ligand by excitation of a RuII→tpphz MLCT. For Ru(tpphz)PtCl2, the transfer of the second electron, which is indispensable for hydrogen evolution at the Pt center, onto the tpphz bridging ligand is impeded by the additional charge localized on the phenazine (phz) moiety.3b Contrary to that, the initial localization of the excited state on the tbbpy ligands in the doubly reduced Ru(tpphz)RhCp* should not adversely affect the turnover at the RhCp* moiety since no third electron is required on the RhI center to promote typical metal-mediated catalysis.19
Resonance Raman experiments upon excitation at 647 nm on the chemically reduced Ru(tpphz)RhCp* (see Figure 2 F) yield identical band pattern as the corresponding experiment on the electrochemically reduced catalysts. Together with the striking similarities of the respectively generated UV/Vis spectra (Figure 2 A and B), this provides clear evidence that both approaches yield the same product, that is, [(tbbpy)2RuII(tpphz)RhICp*]. Furthermore, UV/Vis spectroscopic investigations also confirms the similarity of the electrochemically and photochemically generated doubly reduced Ru(tpphz)RhCp* (Figures 1, 2 A, and S17).
Evaluating the Catalytic Competences of (tbbpy)2RuII(tpphz)RhICp*
The reactivity of RuII(tpphz)RhICp* towards the hydrogenation of N-benzylnicotinamide (BNA+ to BNAH) can be monitored by the increased absorbance of the product around 355 nm (Figure 1 B).6 Here, we generated the “fully charged” RuII(tpphz)RhICp* by chemical reduction with CoCp2. Upon reaction with BNA+ in the dark, formation of BNAH is apparent by an increased absorbance around 355 nm, the loss of absorbance of the RhI-tpphz MLCT band around 650 nm verified the re-oxidation of RhI to RhIII (Figure 1 C). Performing the same reaction under irradiation with an LED at λ=463±12 nm (45 mW cm−2) showed only insignificant changes of the absorption both at 650 and 340 nm (Figure 1 D), that is, no signs for catalytic hydrogenation are observed.
Thus, we conclude that irradiation of the sample obviously impairs the competence of the catalyst and limits its overall efficiency. To identify and trace the deactivation pathway of the fully charged and competent catalytic species during irradiation, transient-absorption experiments will be discussed in the following.
Femtosecond Time-Resolved Transient-Absorption Spectroelectrochemistry
Time-resolved transient-absorption experiments investigating the light-induced electron-transfer dynamics on timescales of sub-ps to ns are performed as TA-SEC measurements using pump wavelengths of 403, 492, and 600 nm. At 403 and 492 nm, both the parent and the doubly reduced Ru(tpphz)RhCp* complex absorb (see Figure 2 B). The parent complex shows transient-absorption features reminiscent of those observed for related [(tbbpy)2Ru(tpphz)MX2]2+ (MX2=PdCl2, PtCl2, PtI2) upon MLCT excitation:2d, 13, 17 An instantaneous bleaching of the 1MLCT band reflects the shift of electron density from the RuII to both tbbpy and tpphz ligands (Figure 3 A). In the latter case, the phenanthroline (phen) moiety serves as the primary electron-density acceptor. The 1-ps process contains contributions from 1MLCT→3MLCT intersystem crossing (ISC), vibrational relaxation, and an inter-ligand charge transfer (ILCT) from the tbbpy moiety to the phenanthroline part of the tpphz ligand (Figures 2 D and S16) (Due to the limited temporal resolution and the spectral congestion of the processes, the individual contributions cannot be resolved). The phenanthroline-centered state relaxes into a phenazine-centered 3MLCT state (τ2=11 ps), which decays with a lifetime of 450 ps (τ3; Figures 3 D and S16). The assignment of the phenazine-centered 3MLCT state is based on the broad absorption of the phenazine radical anion at about 590 nm, which builds up within 20 ps concomitantly with a slight blue-shift of the absorption maximum (Figure 3 A).
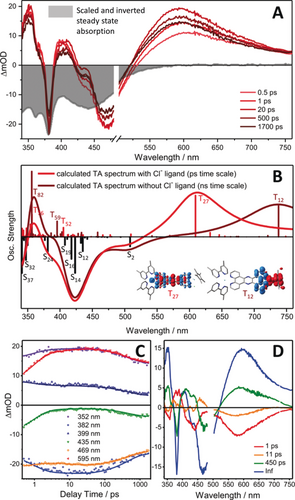
Transient-absorption data including A) experimental and B) calculated transient-absorption spectra at selected delay times, C) transient kinetics at key wavelengths, and D) spectral changes associated with each kinetic process (DAS) for non-reduced Ru(tpphz)RhCp* pumped at 492 nm. For comparison, the inverted (that is, negative) steady-state absorption of the complex Ru(tpphz)RhCp* is also plotted (in gray) and scaled to the largest ground-state bleach signal at 360 nm (A). Inset in (B): Charge-density differences for bright spin-allowed triplet–triplet excitations into intra-ligand State T27 (with Cl−) and into metal-to-ligand charge-transfer state T12 (without Cl−); charge transfer takes place from red to blue.
Further evidence for the origin of the excited-state absorption (ESA) at 590 nm is provided by TD-DFT calculations addressing the absorption by spin-allowed triplet–triplet excitations, which reveal a bright 3IL transition at 609 nm centered on the phenazine moiety (see T27 in Figure 3 B). This absorption subsequently loses intensity, in particular the blue part of the band (Figure 3 A and C).
As a result, the 450-ps component in the decay-associated spectra (DAS) shows no intensity towards the near IR, while the infinite-time spectrum appreciably absorbs at 750 nm (Figure 3 D). This feature indicates electron transfer from the formally reduced phenazine species towards the catalytic RhIII center: TD-DFT reveals that the [(tbbpy)2RuIII(tpphz)RhIICp*] species shows appreciable absorption at 738 nm due to a bright RhII→tpphz 3MLCT (T12), while [(tbbpy)2RuIIItpphz.−RhIIICp*Cl] strongly absorbs at around 600 nm, based on the 3IL excitation into T27 (Figure 3 B). These results explain the spectral shape of the visible excited-state absorption reflected in the DAS of the τ3- and the long-lived component (Figure 3 D).
The excellent match between the experimentally obtained DAS and the calculated spectra indicates not only electron transfer from the photoactive [(tbbpy)2RuII(tpphz)] fragment to the catalytically active Rh center, it also confirms Cl− dissociation on the 450 ps timescale upon metal-centered one-electron reduction.20 Notably, structural reorganization at the reduced RhII center upon Cl− dissociation leads to a linear tpphz-Rh-Cp* geometry in which the RhII center is shielded by steric and electronic factors from geminate recombination with the solvated Cl−, hence leading to the long-lived species and, as a consequence, to the long-lived red-absorbing component in the transient absorption (blue infinite-time component in the DAS, Figures 3 D and S16). In a recent report, this slow decay of the RhII species has been shown to obey second-order kinetics, which was interpreted as a disproportionation reaction yielding RhI and RhIII in the μs-to-ms time range.21
After the two-electron reduction of Ru(tpphz)RhCp* accompanied by Cl− dissociation from the pentamethylcyclopentadienyl-RhIII center,11, 22 the photodynamics of [(tbbpy)2RuII(tpphz)RhICp*], that is, the photoinduced processes in a “fully charged” molecular photocatalyst, are observed (Figure 4). To the best of our knowledge, the data discussed in the following present the first ultrafast-transient-absorption study on an isolated molecular photocatalyst in its fully active electronic configuration.
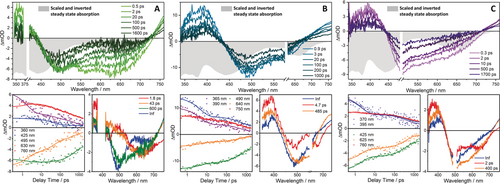
Transient-absorption data including transient absorption spectra at selected delay times, transient kinetics at key wavelengths, and spectral changes associated with each kinetic process (DAS) for doubly reduced Ru(tpphz)RhCp* pumped at A) 403, B) 600, and C) 492 nm. For comparison, the inverted, (that is, negative) steady-state absorption spectra of doubly reduced Ru(tpphz)RhCp* are also plotted (in gray) and scaled to the maximum ground-state bleach signal within the individual graphs.
As discussed in the context of the rR data, excitation of [(tbbpy)2RuII(tpphz)RhICp*] at 403 nm populates a MLCT state in which electron density is shifted to the tbbpy ligands, that is, [(tbbpy)(tbbpy.−)RuIII(tpphz)RhICp*] is formed (Scheme 1). The ESA at 360 nm reflects intra-ligand absorption of the tbbpy radical anion (Figure 4 A). Nonetheless, photoexcitation leads to an instantaneous (within the experimental time resolution) and rather strong bleach signal at 650 nm, which is assigned to a RhI→tpphz MLCT transition based on TD-DFT and UV/Vis-SEC results (Figures 2 B,C and 4 A). This feature thus indicates an interaction between the two metal centers and a hole transfer from the photoexcited RuIII center to the RhI center. This process occurs rapidly, that is, below 500 fs, and leads to the formation of [(tbbpy)(tbbpy.−)RuII(tpphz)RhIICp*]. This state is initially vibrationally hot and cools down with the time constant τ1=1.8 ps. Cooling causes a slight shift of both the ESA maxima at 470 nm and the ΔOD=0 crossing at 725 nm (Figure 4 A). The relaxed [(tbbpy)(tbbpy.−)RuII(tpphz)RhIICp*] reveals a strong ESA above 720 nm, which the calculations assign to a tpphz→RhII-LMCT transition. In addition to the tbbpy.− absorption below 440 nm, the bleaching of the RhI→tpphz MLCT transition at around 630 nm is apparent (Figure 4 A). The strong bleaching of the signal at about 480 nm (RuII center) is not visible in DAS (τ1) and DAS (τ2) but only appears on a 100 ps timescale. On this timescale, associated with τ2=43 ps, inter-ligand tbbpy.−→tpphz electron transfer takes place, that is, [(tbbpy)2RuII(tpphz.−)RhIICp*] is formed (Scheme 1). We hypothesize that the partial bleaching of the signal associated with the Ru center in [(tbbpy)(tbbpy.−)RuII(tpphz)RhIICp*] is superimposed by a tbbpy.−→tpphz excited-state absorption. This renders the net optical-density changes in the spectral region below 500 nm slightly positive. Upon inter-ligand tbbpy.−→tpphz electron transfer, more prominent GSB features below 500 nm are observed, while the bleaching of the signal associated with the Rh center at 650 nm decreases (Figure 4 A). Interestingly, the inter-ligand charge transfer process is relatively slow compared to inter-ligand hopping processes observed in RuII complexes and the non-reduced parent Ru(tpphz)RhCp* (11 ps), which is attributed to the fact that electron density in the RhII center partially extends towards the tpphz-bridging ligand, as indicated by the respective spin density in Figure S15. Hence, the additional negative charge on the phenanthroline fraction of the tpphz ligand coordinating the Rh ion slows down the kinetics of inter-ligand electron transfer towards the bridging ligand. With the characteristic time constant of τ3=600 ps, the excess charge density on the tpphz ligand shifts to the lowest orbital available, that is, from the phenanthroline to the phenazine moiety of the bridging ligand. This is manifested in the comparably low negative ΔOD amplitude at around 630 nm, which stems from the superposition of the Rh-center ground-state bleach and the excited-state absorption of the reduced tpphz ligand, more specifically the reduced central phenazine unit (DAS, Figure 4 A).23 The resultant [(tbbpy)2RuII(phen-phz.−-phen)RhIICp*], bearing the electron in the central phenazine moiety, forms the long-lived species which decays back to the ground state with a time constant beyond the experimentally accessible time range (Scheme 1).
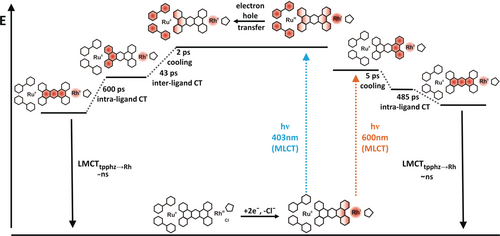
Schematic representation of the proposed photophysical pathways for the doubly reduced photocatalyst Ru(tpphz)RhCp* upon photoexcitation at 403 and 600 nm. At 403 nm, a MLCT from RuII to the tbbpy ligand occurs, after which an electron hole is transferred on a sub-500-fs timescale, reducing RuIII and oxidizing the RhI center. This process is followed by an inter-ligand transition to tpphz, finally decaying by an intra-ligand charge transfer from the phenanthroline to the phenazine fragment of the bridging ligand, followed by a MLCT to the ground state. In contrast, at 600-nm excitation, a MLCT from RhI to tpphz occurs which decays via an intra-ligand charge transfer to the ground state. Here, the Ru center and the tbbpy ligands are not involved in the photodynamic processes.
The slow ns-decay component observed in the TA-SEC data upon 403-nm excitation corresponds to the intramolecular electron transfer of the second electron onto a mono-reduced catalytic RhII center. This electron-transfer step is apparently much slower than the corresponding first electron transfer in the RhIII→RhII reduction (450 ps, see Figure 4 A). Since the electrochemical analysis showed a potential inversion of the RhIII/RhII and RhII/RhI reduction, the slowed-down electron transfer must be attributed to the change in the relative orientation between the tpphz bridging ligand and the Cp* ligand, which results in a kinetic barrier for the electron-transfer process.
As concluded from the TA measurements and the quantum-chemical calculations, a perpendicular arrangement between the tpphz bridge and the Cp* ligand also exists in the [(tbbpy)2RuII(tpphz)RhIICp*] state (Figure S16). Similar to the RhI species, electron-density redistribution from the strong donor ligand Cp* over the connecting RhII core to the phenanthroline moiety of the tpphz ligand might establish an electronic barrier for the electron localized at the phenazine moiety which drastically reduces the transfer kinetics of the second electron at the catalytic unit. 600-nm excitation of the “fully charged” Ru(tpphz)RhCp* photocatalysts allows for a cross-validation of the model put forward before.
Excitation within the reduction-induced absorption band (see Figure 2 B) leads to a charge-density shift associated with a RhI→tpphz MLCT transition. TD-DFT calculations show that the excess charge density is localized on the phenanthroline part of the bridging ligand that coordinates the RhI ion. Hence, 600-nm excitation of the doubly reduced Ru(tpphz)RhCp* catalyst initially leads to a vibrationally hot [(tbbpy)2RuII(phen-phz-phen.−)RhIICp*] state (Scheme 1). The subsequent dynamics are characterized by a 4.7- and a 485-ps process (DAS, Figure 4 B), which lead to the population of a molecular species that outlives the experimentally accessible delay-time window of 1.8 ns. The fastest component, τ1=4.7 ps, is spectrally characterized by a blue-shift of the ΔOD=0 crossing at around 680 nm and the disappearance of a negative shoulder at the red-edge of the RhI-ground-state bleach (Figure 4 B). Hence, it is associated with a cooling of the [(tbbpy)2RuII(phen-phz-phen.−)RhIICp*] state. However, it should be noted that the excess electron density on the tpphz ligand is still considered to be localized on the phenanthroline moiety. Only during the process associated with τ2=485 ps, the charge density relaxes to the central phenazine part of the tpphz ligand by forming the species [(tbbpy)2RuII(phen-phz.−-phen)RhIICp*] (Scheme 1). This species is found to be long-lived on the time scale of the femtosecond-transient-absorption experiment.
It should be pointed out that the formation of [(tbbpy)2RuII(phen-phz.−-phen)RhIICp*] occurs with a charge-density shift from the RhI fragment upon 600-nm excitation with a time constant of 485 ps, and from the RuII fragment upon 403-nm excitation with a time constant of 600 ps (Figure 4 A,B). The similarity of the characteristic timescales reflects the apparent energetic symmetry of the tpphz ligand even though it coordinates two different metal ions. In either of these cases, the phenanthroline→phenazine charge-density shift is spectrally manifested in an apparent decrease of the ground-state bleach in the red part of the ΔOD<0 band. This is due to the fact that the phz.− fragment strongly absorbs at around 550 nm, that is, partially overriding the ground-state bleach in the same spectral region (Figures 4 A,B).23
Photoexcitation of doubly reduced Ru(tpphz)RhCp* at 492 nm leads to similar transient-absorption features obtained with 403-nm excitation (Figure 4 C). In agreement with rR-SEC at 473 nm, a RuII→tbbpy MLCT state is initially populated (Figures 2 E and 4 C). Additionally, a RhI→tpphz MLCT transition occurs, since both absorption features are spectrally broad and overlapping. Consequently, two overlapping decay processes from both MLCT transitions are observed in parallel. Therefore, the TA spectra obtained at 403- and 492-nm excitation are qualitatively similar, but their interpretation is significantly complicated. Therefore, the data was not analyzed in detail, because too many decay constants would be required, rendering an in-depth kinetic analysis unreliable.
The combined catalytic, photophysical, electrochemical and theoretical data paint a very similar picture, that is, photoexcitation of the “fully charged” Ru(tpphz)RhCp* complex containing a RhI ion leads to the photochemical formation of a RhII intermediate. The same light required for the photochemical generation of the catalytically competent RhI state thus leads to its deactivation by MLCT formation. This is directly reflected by the absence of catalytic activity of the intermediate RuII(tpphz)RhICp* under irradiation. The photoinduced discharging produces a relatively long-lived RhII state describing, for the first time, the catalytic inactivity of a mono-reduced (N,N)RhCp* complex, that is, the RhII state, towards nicotinamide reduction. This is particularly important because the electrochemical properties of virtually all (N,N)RhCp* complexes only permit experimental access to the directly generated RhI state during metal-centered reduction. Based on our mechanistic conclusions, we suggest — in the absence of available protons — controlling the overall catalytic process by sequential photochemical RhI generation and thermal consumption of RhI coupled to substrate conversion. Utilizing this approach, BNAH formation was successfully implemented (see Figure S19).
Conclusion
The elucidation of multi-electron reaction pathways, for example, in hydrogen-evolving photocatalysis, requires the identification and dynamic monitoring of intermediates, which can be mimicked by sequential electroreduction. To the best of our knowledge, we presented, for the first time, the results of in-situ early-time photodynamics of an isolated, molecular photocatalyst in its active electronic configuration. Pulsed laser excitation of doubly reduced Ru(tpphz)RhCp* at either 403 or 600 nm leads to a charge-density shift towards the central phenazine part of the bridging tpphz ligand, irrespective of the fact that completely different states are initially populated. At 403 nm, a RuII→tbbpy MLCT transition occurs, while at 600 nm, a RhI→tpphz MLCT state is populated, as confirmed by rR-SEC and TD-DFT. The long-lived radical anion [(tbbpy)2RuII(tpphz.−)RhIICp*] in the described relaxation cascade is possibly a potent precursor for an additional chemical deactivation pathway of the catalyst.24 The investigations towards the effect of blue-light irradiation of the catalytic reaction mixture containing BNA+ clearly showed that once the doubly reduced Ru(tpphz)RhCp* state is formed, photoexcitation impedes the catalytic hydrogenation of BNA+ to BNAH. This is in full agreement with the results of the detailed spectroscopic and theoretical investigation which point to the formation of an inactive RhII intermediate upon photoexcitation. The excitation-induced charge redistribution in the doubly reduced Ru(tpphz)RhCp* can be formally viewed as a RhI→RhII discharging process leading to the inactivation of the catalytic center. However, the actual “H”-transferring species in RhCp* catalysts is either a RhI(Cp*H)19b, 25 or a RhIII(Cp*)H19a, 26 unit, formed upon fast26 oxidative addition of a proton to the RhICp* moiety. Since this hydride-transferring agent does not exhibit a red absorbance,19b it is possible in these systems to escape the potential visible-light-driven inactivation of catalysis by working in sufficiently acidic media.
With respect to future applications of molecular catalysts using the whole solar spectrum for light-driven catalysis, the detected MLCT transitions from the reductively fully activated catalytic center back to the bridging ligand presumptively represent an activity-limiting step. It is clear that a more careful design of the ligand moiety supporting the catalytic center is crucial for supporting sufficiently high activity. The presented results clearly show that the design of photocatalytically active systems will benefit from close interactions between spectroscopy, theory, and synthesis, leading to the spectroscopy-aided design of next generation catalysts.
Experimental Section
Ru(tpphz)RhCp* was synthesized as described in the literature.5 UV/Vis-SEC, rR-SEC, and electrochemical measurements were performed as described in detail in the Supporting Information. A custom-built setup was utilized to acquire fs-TA data (Pascher Instruments AB).28 Comprehensive experimental and computational details and methods can be found in the Supporting Information.
Acknowledgements
This work was financially supported by the German Science Foundation (DI1517/11-1 and CATALIGHT CRC/RR 234, projects A1 and C5, Projektnummer 364549901). We thank the Thuringian State Government for financial support within the ACP Explore project. A.K.M. acknowledges financial support from the FCI (via a Chemiefonds-Stipendium). Y.Z. acknowledges support by the German Academic Exchange Service. K.M.Z. acknowledges support from the Konrad Adenauer foundation and the German Academic Scholarship Foundation.
Conflict of interest
The authors declare no conflict of interest.




