Persistent Organic High-Spin Trinitrenes
Abstract
The septet ground state trinitrenes 1,3,5-trichloro-2,4,6-trinitrenobenzene and 1,3,5-tribromo-2,4,6-trinitrenobenzene were isolated in inert (Ar, Ne, and Xe) as well as reactive matrices (H2, O2, and H2O) at cryogenic temperatures. These trinitrenes were obtained in high yields by UV photolysis of the corresponding triazides and characterized by IR and UV/Vis spectroscopy. The trinitrenes, despite bearing six unpaired electrons, are remarkably unreactive towards molecular oxygen and hydrogen and are persistent in water ice up to 160 K where the water matrix starts to sublime off.
Organic magnetic materials are of large current interest and studied in many laboratories.1 The properties of organic magnets are quite different from conventional metallic magnets: lightweight, transparent, flexible or even liquid magnets could be realized with properties switchable by external stimuli, such as light or ligands. The downside of these materials is their frequently low chemical, thermal, or photochemical stability.
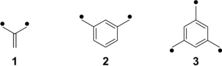
One approach towards the synthesis of organic magnets is to use molecular high-spin systems with many spin centers as building blocks. Although magnetism is strictly a property of the solid state, the term “molecular magnet” is widely used for these systems. These building blocks are characterized by strong ferromagnetic coupling between the radical centers. Most of the building blocks for constructing high-spin molecules are based on non-Kekulé molecules such as trimethylenemethane (1), m-xylylene (2), or 1,3,5-trimethylenebenzene (3). A number of very high-spin polyradicals (e.g. with S=5000) have been published by Rajca et al.2, 3 Diarylcarbenes are molecules with triplet ground states that have been used by Iwamura et al. as units to construct “superparamagnetic” polycarbenes.4, 5 While this approach was successfully used to synthesize organic molecules in very high-spin states, the drawback of these systems is their high chemical reactivity.
Arylnitrenes, which in general show robust triplet ground states with large S–T splittings, have been much less considered as basic units for the construction of high-spin molecules than carbenes. The advantage of arylnitrenes is that there are convenient precursors available for their synthesis, and that the magnetic interaction between the unpaired electrons is very large. The zero-field splitting (zfs) parameter D of arylnitrenes is in the order of 1 cm−1, which is about three times larger than that of triplet arylcarbenes (typically 0.2–0.4 cm−1).1 The disadvantage is the high photolability of many arylnitrenes. Arylnitrenes are generally synthesized by photolysis of the corresponding arylazides, which in many cases produces only low yields of the nitrenes and large amounts of rearranged products, in particular azirines and cyclic ketenimines. Thus, the photolysis of matrix-isolated phenylazide (4) produces phenylnitrene (5) in low yields, because 5 rearranges to azirine (6) that subsequently forms azacyclohexatetraene (7; Scheme 1).6, 7 Substituents in the ortho position of 5, in particular F, Cl, and Br, increase the yields of the nitrene.
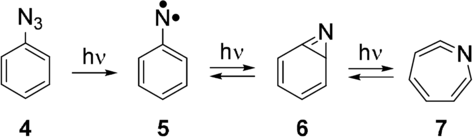
Photochemical generation and rearrangement of phenylnitrene (5).
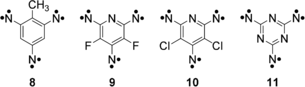
High-spin arylnitrenes are obtained by arranging two or three nitrene centers in meta position of an aromatic core, which results in ferromagnetically coupled dinitrenes and trinitrenes with quintet and septet ground states, respectively.8 A number of trinitrenes (e.g. 8–11) have been studied by the very sensitive electron paramagnetic resonance (EPR) spectroscopy.9-13 Only a few of these high-spin nitrenes could be synthesized in high enough yields to be characterized by IR9, 14, 15 or UV/Vis spectroscopy16 in inert gas matrices. In most cases, the yield of trinitrenes is low due to incomplete photolysis of the precursor leading to mono- or dinitrenes, or because of secondary photoreactions that produce rearranged products such as azirines, ketenimines or nitriles.15, 17, 18
Experimental and theoretical studies suggest that undesirable rearrangements of aryl di- and trinitrenes are effectively suppressed by blocking the positions adjacent to the nitrene units with halogen atoms.19, 20 Photolysis of 1,3,5-triazido-2,4,6-trichlorobenzene (12 a) and its bromo-analogue 12 b should therefore provide high yields of the septet trinitrenes 15 a and 15 b, respectively (Scheme 2). Indeed, EPR (X-band 9 GHz) studies by Misochko et al., concluded that septet trinitrene 15 a is the major paramagnetic product of the photolysis of precursor 12 a in Ar matrices at 15 K.21 Similarly, trinitrene 15 b was also produced in low-temperature organic glasses. In this case, the more sensitive W-band 94 GHz EPR technique allowed to identify in addition to 15 b the triplet 13 b and quintet 14 b partially photolyzed species.22 Herein, we report the isolation and spectroscopic characterization (IR and UV/Vis) of septet trinitrenes 15 a and 15 b in both inert gas (Ar, Ne, and Xe) and reactive host (H2, O2, and H2O) low-temperature matrices.
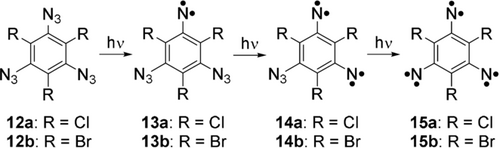
Photochemical synthesis of septet trinitrenes 15 a and 15 b.
Matrix isolated 1,3,5-triazido-2,4,6-trichlorobenzene (12 a) was irradiated with UV light (λ=365 nm) for 4 h in Ar matrices at 3 K. The clean IR spectrum of the product containing five signals indicates the formation of a highly symmetric photoproduct which was identified as 1,3,5-trichloro-2,4,6-trinitrenobenzene (15 a) in its septet spin state (Figure 1 traces a, b, Table 1). The IR bands found at 1330, 1257, 1131, 783, and 691 cm−1 are in good agreement with the vibrations calculated at the M06-2X/aug-cc-pVTZ level of theory (Figure 1 trace b, Table 1). Trinitrenes generally exhibit large energy gaps between the septet ground state and the next higher-energy states (quintet, triplet, and open-shell singlet), hence are properly described by single-determinant methods as density functional theory (DFT).23 Neither remaining precursor 12 a, nor the intermediates triplet 13 a or quintet 14 a could be detected at the end of the photolysis (Figure S1 in Supporting Information). Typical photoproducts of nitrenes, such as azirines and cyclic ketenimines were not found either.20 The stability of trinitrene 15 a contrasts that of the related trinitrene 10, which could only be generated in yields too low for the complete assignment of the IR spectrum.9
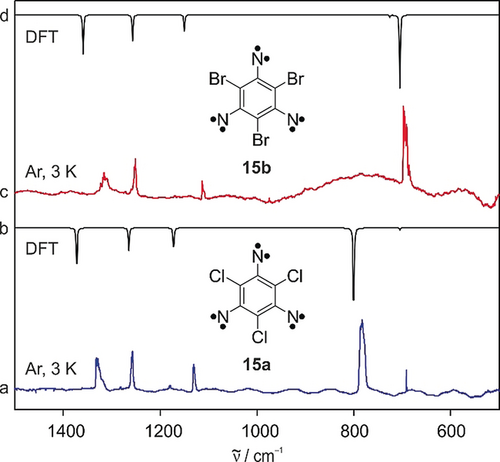
IR spectra showing 15 a and 15 b isolated in Ar matrices. a) IR spectrum obtained after irradiating triazide 12 a with λ=365 nm in Ar at 3 K. b) IR spectrum of septet trinitrene 15 a calculated at the M06-2X/aug-cc-pVTZ level of theory. c) IR spectrum obtained after irradiating triazide 12 b with λ=405 nm in Ar at 3 K. d) IR spectrum of septet trinitrene 15 b calculated at the M06-2X/aug-cc-pVTZ level of theory.
Trinitrene |
Argon[a] |
Calculated[b] |
Symmetry |
Assignment |
||
|---|---|---|---|---|---|---|
|
|
Irel.[c] |
|
Irel.[c] |
|
|
15 a |
691 |
0.03 |
705 |
0.04 |
A2′′ |
ring def. (oop) |
783 |
1.00 |
800 |
1.00 |
E′ |
C−Cl str. |
|
1131 |
0.15 |
1173 |
0.26 |
E′ |
ring def. (ip) |
|
1257 |
0.22 |
1265 |
0.24 |
E′ |
C−N str. |
|
1330 |
0.43 |
1373 |
0.50 |
E′ |
C−C str. |
|
|
|
|
|
|
|
|
15 b |
686 |
0.08 |
726 |
0.07 |
A2′′ |
ring def. (oop) |
695 |
1.00 |
706 |
1.00 |
E′ |
C−Br str. |
|
1113 |
0.13 |
1150 |
0.22 |
E′ |
ring def. (ip) |
|
1251 |
0.32 |
1257 |
0.36 |
E′ |
C−N str. |
|
1316 |
0.50 |
1359 |
0.54 |
E′ |
C−C str. |
|
- [a] Ar matrix at 3 K. [b] Calculated at the M06-2X/aug-cc-pVTZ level of theory. [c] Relative intensities based on the strongest absorption.
Photolysis (λ=405 nm, 15 h) of matrix-isolated (Ar, 3 K) 1,3,5-triazido-2,4,6-tribromobenzene (12 b) produces high yields of 1,3,5-tribromo-2,4,6-trinitrenobenzene (15 b) in its septet ground state with prominent IR signals at 1316, 1251, 1113, 695, and 686 cm−1 (Figure 1 traces c, d, Table 1). The final spectrum is free from precursor 12 b and intermediates 13 b and 14 b. Most of the IR signals of 15 b are red-shifted by only a few wavenumbers (5–20 cm−1) with respect to the structurally related 15 a, while the C−X stretching vibration of the heavier halogen is red-shifted by 90 cm−1, due to higher mass and weaker bond strength. Such large red-shift causes the collapsing of the weaker A2′′ mode into the broad and intense C−Br stretching mode in trinitrene 15 b.
Irradiation (λ=365 nm, for 15 min) of triazide 12 a in Ne matrices results in the formation of weak IR bands at 1477, 1348, 1229, and 808 cm−1, which are assigned to quintet dinitrene 14 a (Figure S2, Table S1 in the Supporting Information). These signals reach maximum intensity after 1 h of irradiation but gradually decay during extended irradiation while trinitrene 15 a is formed concomitantly.
Triazide 12 a, matrix-isolated in Ar at 8 K, shows a broad and strong absorption centered at 246 nm (Figure 2 trace a). Irradiation of the matrix with λ=365 nm for 3 h results in the depletion of all bands assigned to 12 a and the appearance of a new set of bands assigned to trinitrene 15 a (Figure 2 traces b–d, Table S2). The UV/Vis spectrum of 15 a shows a broad, structured band between 345 and 310 nm as well as bands with maxima at 289, 283, 237, 221, and 204 nm. The UV/Vis absorptions are reasonably reproduced by a time-dependent DFT calculation at the (TD)M06-2X/aug-cc-pVTZ level of theory. The broad absorption between 310 and 345 nm shows a vibrational fine structure and is assigned to a nsp→py transition (calculated at 338 nm) localized at the nitrogen atom. This transition is very characteristic for nitrenes.24
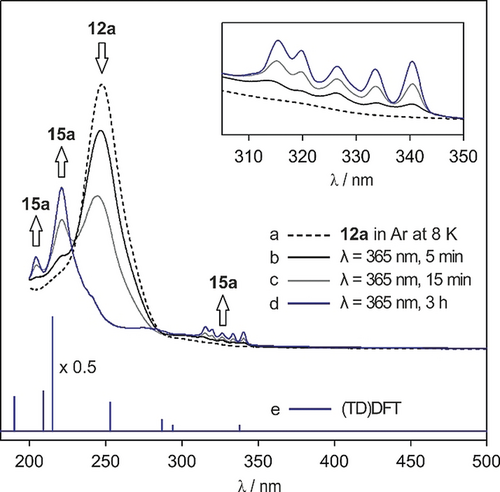
UV/Vis spectra showing the formation of 15 a in Ar matrices. a) UV/Vis spectrum of triazide 12 a in Ar at 8 K. b)–d) UV/Vis spectra obtained after irradiating 12 a with λ=365 nm for 5, 15 min, and 3 h, respectively. e) Electronic transitions of septet trinitrene 15 a calculated at the (TD)M06-2X/aug-cc-pVTZ level of theory. Inset: expansion showing the intensity increase of the signals of 15 a at 345–310 nm upon irradiation with λ=365 nm.
Dinitrenes show characteristic absorptions around 450–500 nm.25 From the absence of such absorptions in the spectra we conclude that dinitrene 14 a is not formed in detectable quantities, in accordance with the results from the IR experiments in Ar matrices.
In additional experiments the UV/Vis and IR spectra of 15 a were recorded from the same matrix. All UV/Vis and IR absorptions assigned to 15 a show the same behaviour during irradiation of the precursor 12 a and UV photolysis (λ=254 nm) of 15 a which confirms the assignment of these bands to 15 a (Figure S3).
Photolysis (λ=405 nm, 15 h) of triazide 12 b in Ar at 8 K produces trinitrene 15 b in high yields (Figure S4, Table S2). The UV/Vis spectrum shows intense bands at 233 and 218 nm and a broad band between 355 and 320 nm, similar to that of 15 a.
Since applications of trinitrenes as molecular magnets might be hampered by rapid reactions with environmental oxygen, we studied 15 a in O2 matrices and O2-doped xenon matrices. UV photolysis (λ=365 nm) of 12 a in solid O2 at 3 K generates trinitrene 15 a in high yields. No photochemical reaction of 15 a is observed under these conditions. The trinitrene is thermally stable in solid oxygen up to 20 K when O2 starts subliming off (Figure 3 trace b, Figure 4 trace b). To achieve higher temperatures, experiments were carried out in 5 % O2-doped Xe matrices. O2 remains trapped in these matrices up to the beginning of rapid sublimation at temperatures above 60 K for Xe matrices. Annealing of such matrices containing 15 a for 12 h at 50 K did not lead to any reaction, indicating the remarkable stability of trinitrene 15 a towards molecular oxygen. While phenylnitrene 5 is also stable in O2 matrices at 20 K, it rapidly reacts in O2-doped Xe at 50 K. 25b Thus, despite bearing three nitrene units, 15 a is even less reactive towards O2 than 5.
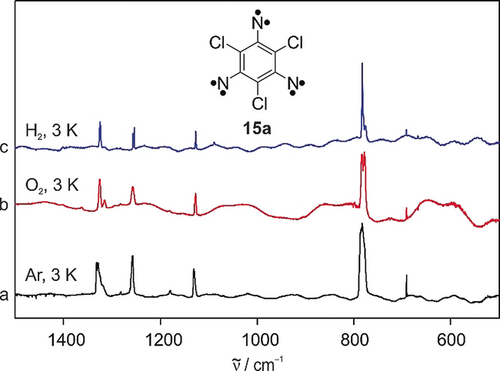
IR spectra showing the isolation of 15 a in reactive matrices. a)–c) IR spectra obtained after irradiating triazide 12 a with λ=365 nm in Ar (a), O2 (b), and H2 (c) at 3 K.
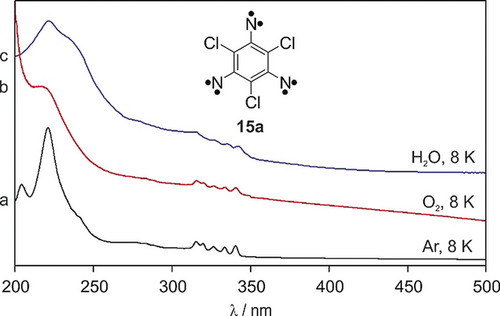
UV/Vis spectra showing the isolation of 15 a in reactive matrices a)–c) IR spectra obtained after irradiating triazide 12 a with λ=365 nm in Ar (a), O2 (b), water ice (c) at 8 K. Trinitrene 15 a is stable in water ice matrices up to 160 K, at this point H2O starts subliming.
The thermal reaction between T-5 and 3O2 proceeds via a triplet transition state to triplet nitroso O-oxide T-16, which subsequently relaxes by intersystem crossing (ISC) to S-16.26, 27 A small activation barrier of 4.3±0.5 kcal mol−1 was determined for this reaction by laser flash photolysis (LFP) experiments, which explains the lack of reactivity at temperatures below 20 K and the slow reaction at 50 K.28 The rate constants of reactions of para-substituted phenylnitrenes with 3O2 decrease with electron withdrawing substituents (k > kH > k
> kH > k ).29
).29
The reaction of T-5 with 3O2 to T-16 was calculated (M06-2X/aug-cc-pVTZ) to be slightly endothermic with 2.5 kcal mol−1 while the formation of S-16 is exothermic with −5.1 kcal mol−1 (Scheme 3), which rationalizes the experimental data.28 The chlorination of the ortho and para positions as in T-5 a increases the energy of the triplet nitroso O-oxide T-17 a to 10 kcal mol−1 and makes the formation of S-17 a slightly endothermic (2.4 kcal mol−1), and thus this nitrene is expected to be even less reactive towards 3O2.
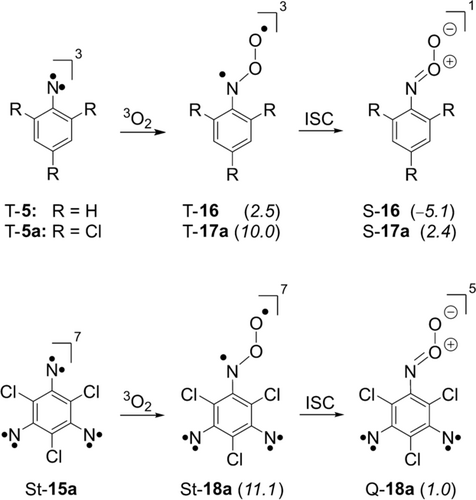
Thermochemistry of the oxidation of triplet nitrenes T-5 and T-5 a, and septet trinitrene St-15 a calculated at the M06-2X/aug-cc-pVTZ level of theory. Arylnitroso O-oxides 16 and 17 a are optimized in their singlet (S) and triplet (T) states, whereas dinitrenophenylnitroso O-oxide 18 a is optimized in its quintet (Q-18 a) and septet (St-18 a) states. Relative energies to the nitrene and the septet trinitrene are shown in parenthesis and are given in kcal mol−1.
In analogy, we calculated the reaction of septet St-15 a with 3O2 to give the corresponding septet nitroso O-oxide St-18 a to be endothermic by 11.1 kcal mol−1. Septet St-18 a is an excited state that is expected to interconvert rapidly to its quintet ground state Q-18 a in an overall almost thermoneutral reaction (1 kcal mol−1). At the M06-2X/aug-cc-pVTZ level of theory the NOO moiety in quintet Q-18 a has a zwitterionic character with a short N−O bond of 1.31 Å, whereas septet St-18 a shows a longer N−O bond of 1.40 Å and a diradical character. The high energy of both the septet and quintet nitroso O-oxide leads to the lack of reactivity of 15 a towards molecular oxygen.
UV photolysis (λ=365 nm) of triazide 12 a in solid H2 at 3 K results in the formation of trinitrene 15 a in high yields, while no hydrogenated product can be observed (Figure 3 trace c). DFT calculations (M06-2X/aug-cc-pVTZ) suggest that the H-abstraction to form the sextet dinitrenoaminyl radical is endothermic by 12.6 kcal mol−1, and the activation barrier for this reaction is, with 22.7 kcal mol−1, prohibitively high under low temperature conditions. The endothermicity of the H-abstraction results from the lower bond dissociation energy of N−H compared to H−H.30 This is in contrast to the chemistry of triplet arylcarbenes which insert into H2 even at temperatures as low as 3 K.31
Low-density amorphous water is a polar matrix that has proven to be a suitable medium to stabilize reactive species.32, 33 Triazide 12 a was isolated in amorphous water ice at 8 K and photolyzed (λ=365 nm) until all UV/Vis bands of the precursor disappeared. The spectrum obtained matches that of 15 a in Ar and O2 matrices, only the linewidths are wider in water ice than in the other matrices (Figure 4, trace c). During warm up of ice matrices containing 15 a, the UV/Vis spectrum of 15 a could be followed up to 160 K when the matrix rapidly degraded. This demonstrates the high thermal stability and low reactivity of trinitrene 15 a.
In summary, the two trinitrenes 15 a and 15 b are obtained in very high yields by photolysis of their triazide precursors 12 with only traces of mononitrenes 13 or dinitrenes 14 as contaminants (Scheme 2). Rearranged products are below the detection limits. Clearly, 13 and 14 are more labile under the photolysis conditions than 12 and therefore do not accumulate during partial photolysis. The trinitrenes 15 are remarkably unreactive towards oxygen and are not even oxygenated during the precursor 12 photolysis. Low reactivity towards 3O2 is a prerequisite for many potential applications. The lifetimes of the trinitrenes 15 are presumably limited by dimerizations and oligomerizations to produce highly substituted nitreno-azobenzenes. Consequently, immobilization of 15 in water ice results in a stabilization as long as the diffusion of the trinitrenes is suppressed. The high yields and stability of trinitrenes 15 combined with the large magnetic interaction between the three nitrene units makes these molecules promising building blocks for the design of organic magnetic materials.
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—EXC-2033—Projektnummer 390677874. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.



 [cm−1]
[cm−1] [cm−1]
[cm−1]
