Total Structure and Electronic Properties of the Gold Nanocrystal Au36(SR)24†
Chenjie Zeng
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Huifeng Qian
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorTao Li
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Gao Li
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorProf. Nathaniel L. Rosi
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Bokwon Yoon
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorDr. Robert N. Barnett
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorProf. Robert L. Whetten
School of Chemistry & Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorProf. Uzi Landman
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorCorresponding Author
Prof. Rongchao Jin
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)Search for more papers by this authorChenjie Zeng
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Huifeng Qian
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorTao Li
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Gao Li
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorProf. Nathaniel L. Rosi
Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15213 (USA)
Search for more papers by this authorDr. Bokwon Yoon
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorDr. Robert N. Barnett
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorProf. Robert L. Whetten
School of Chemistry & Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorProf. Uzi Landman
School of Physics, Georgia Institute of Technology, Atlanta, GA 30332 (USA)
Search for more papers by this authorCorresponding Author
Prof. Rongchao Jin
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)
Department of Chemistry, Carnegie Mellon University, Pittsburgh, PA 15213 (USA)Search for more papers by this authorR.J. thanks financial support by the Air Force Office of Scientific Research under AFOSR Award No. FA9550-11-1-9999 (FA9550-11-1-0147) and the Camille Dreyfus Teacher-Scholar Awards Program. The work of B.Y., R.N.B., and U.L. was supported by the Office of Basic Energy Sciences of the US Department of Energy under Contract No. FG05-86ER45234, and in part by the Air Force Office of Scientific Research (AFOSR). Calculations were performed at the Georgia Tech Center for Computational Materials Science. We thank Dr. Zhongrui Zhou for assistance in ESI-MS analysis.
Graphical Abstract
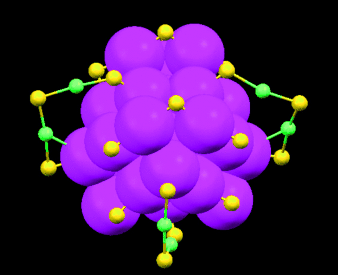
Kleines Goldstück: In der Struktur eines Au36(SR)24-Nanoclusters (siehe Bild) stößt man unerwartet auf einen kubisch-flächenzentrierten tetraedrischen Au28-Kern (rosa), dessen Umhüllung eine Kombination von Bindungsmodi zeigt und vier Klammerliganden sowie zwölf verbrückende Thiolate (gelb) umfasst. Dieses schützende Netz verleiht dem Cluster hohe Stabilität.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201207098_sm_miscellaneous_information.pdf504.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. A. Mirkin, R. L. Letsinger, R. C. Mucic, J. J. Storhoff, Nature 1996, 382, 607.
- 2M. Brust, M. Walker, D. Bethell, D. J. Schiffrin, R. Whyman, J. Chem. Soc. Chem. Commun. 1994, 801.
- 3H. Wu, H. Zhu, J. Zhuang, S. Yang, C. Liu, Y. C. Cao, Angew. Chem. 2008, 120, 3790; Angew. Chem. Int. Ed. 2008, 47, 3730.
- 4R. L. Whetten, J. T. Khoury, M. M. Alvarez, S. Murthy, I. Vezmar, Z. L. Wang, P. W. Stephens, C. L. Cleveland, W. D. Luedtke, U. Landman, Adv. Mater. 1996, 8, 428.
- 5Y. Shichibu, Y. Negishi, H. Tsunoyama, M. Kanehara, T. Teranishi, T. Tsukuda, Small 2007, 3, 835.
- 6R. Jin, Y. Zhu, H. Qian, Chem. Eur. J. 2011, 17, 6584.
- 7R. L. Whetten, R. C. Price, Science 2007, 318, 407.
- 8P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell, R. D. Kornberg, Science 2007, 318, 430.
- 9M. W. Heaven, A. Dass, P. S. White, K. M. Holt, R. W. Murray, J. Am. Chem. Soc. 2008, 130, 3754.
- 10M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz, R. Jin, J. Am. Chem. Soc. 2008, 130, 5883.
- 11H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer, R. Jin, J. Am. Chem. Soc. 2010, 132, 8280.
- 12C. L. Cleveland, U. Landman, T. G. Schaaff, M. N. Shafigullin, P. W. Stephens, R. L. Whetten, Phys. Rev. Lett. 1997, 79, 1873.
- 13J. Akola, M. Walter, R. L. Whetten, H. Häkkinen, H. Grönbeck, J. Am. Chem. Soc. 2008, 130, 3756.
- 14Y. Pei, Y. Gao, X. C. Zeng, J. Am. Chem. Soc. 2008, 130, 7830.
- 15G. Schmid, Chem. Soc. Rev. 2008, 37, 1909.
- 16H. Qian, Y. Zhu, R. Jin, Proc. Natl. Acad. Sci. USA 2012, 109, 696.
- 17E. G. Mednikov, M. C. Jewell, L. F. Dahl, J. Am. Chem. Soc. 2007, 129, 11619.
- 18C. Femoni, M. C. Iapalucci, G. Longoni, S. Zacchini, S. Zarra, J. Am. Chem. Soc. 2011, 133, 2406.
- 19H. Schnöckel, Chem. Rev. 2010, 110, 4125.
- 20H. Qian, Y. Zhu, R. Jin, ACS Nano 2009, 3, 3795.
- 21R. C. Price, R. L. Whetten, J. Am. Chem. Soc. 2005, 127, 13750.
- 22P. R. Nimmala, A. Dass, J. Am. Chem. Soc. 2011, 133, 9175.
- 23D. M. P. Mingos, J. Chem. Soc. Dalton Trans. 1996, 561.
- 24N. K. Chaki, Y. Negishi, H. Tsunoyama, Y. Shichibu, T. Tsukuda, J. Am. Chem. Soc. 2008, 130, 8608.
- 25D. Stellwagen, A. Weber, L. G. Bovenkamp, R. Jin, H. Bitter, C. S. S. R. Kumar, RSC Adv. 2012, 2, 2276.
- 26Z. Wu, J. Suhan, R. Jin, J. Mater. Chem. 2009, 19, 622.
- 27The first-principles molecular dynamics (FPMD) method that we use has been formulated for treating neutral and charged systems, see R. N. Barnett, U. Landman, Phys. Rev. B 1993, 48, 2081. The method, which can be used for structural optimization using a conjugate-gradient-like relaxation, as well as for simulations of the dynamics of the nuclear motions evolving on the concurrently calculated Born–Oppenheimer potential energy surface, calculates the electronic structure by employing the Kohn–Sham density-functional theory (DFT) with the use of a plane wave-basis (62 Ry kinetic energy cutoff), in conjunction with soft pseudopotentials, (after N. Troullier, J. L. Martins, Phys. Rev. B 1991, 43, 19, with those for gold including scalar-relativistic corrections) and the Perdew–Burke–Ernzerhof (PBE) functional ( J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865) in the generalized gradient approximation (GGA) to the exchange-correlation energy.
- 28B. Yoon, P. Koskinen, B. Huber, O. Kostko, B. von Issendorff, H. Hakkinen, M. Moseler, U. Landman, ChemPhysChem 2007, 8, 157.
- 29Recall the theoretical tetrahedral Au28-core in [Au44(SR)28]2− (see D. Jiang, M. Walter, J. Akola, J. Phys. Chem. C 2010, 114, 15883) and the tetrahedral structure of the bare Au20 gas-phase cluster (see J. Li, X. Li, H. J. Zhai, L.-S. Wang, Science 2003, 299, 864). Additionally, see the structure determination of Au20 and Au16 (a t-Th subunit of the 28-atom kernel described here, see Figure 3 and 4) using combined electron scattering and first-principles calculations in X. Xing, B. Yoon, J. H. Parks, U. Landman, Phys. Rev. B 2006, 74, 165423 and also Ref. [28].
- 30Y. Pei, Y. Gao, N. Shao, X. C. Zeng, J. Am. Chem. Soc. 2009, 131, 13619.
- 31P. Maksymovych, O. Voznyy, D. B. Dougherty, D. C. Sorescu, J. T. Yates, Jr., Prog. Surf. Sci. 2010, 85, 206.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.