A Remarkable Organometallic Transformation on a Cage-Incarcerated Dinuclear Ruthenium Complex†
Correction(s) for this article
-
Berichtigung: A Remarkable Organometallic Transformation on a Cage-Incarcerated Dinuclear Ruthenium Complex
- Volume 125Issue 8Angewandte Chemie
- pages: 2201-2201
- First Published online: February 13, 2013
Shinnosuke Horiuchi
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Takashi Murase
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Fujita
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorShinnosuke Horiuchi
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Takashi Murase
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Fujita
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorThis research was supported by the CREST project of the Japan Science and Technology Agency (JST) and KAKENHI (24000009), MEXT (Japan).
Graphical Abstract
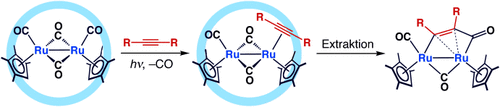
Unter Verschluss: Die Photosubstitution eines CO-Liganden durch ein Alkin auf einem zweikernigen Ru-Carbonyl-Komplex in einem selbstorganisierten Käfig verläuft ohne Spaltung der photolabilen Ru-Ru-Bindung. Der resultierende Ru-Alkin-π-Komplex ist ein Reaktionsintermediat, das im Käfig stabilisiert wird. Außerhalb des Käfigs kann der π-Komplex durch intramolekulare CO-Insertion weiter zu einem Diruthenacyclopentenon reagieren (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201206325_sm_miscellaneous_information.pdf8.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. D. Adams, F. A. Cotton, Catalysis by Di- and Polynuclear Metal Cluster Complexes, Wiley-VCH, New York, 1998;
- 1bB. C. Gates, L. Guzei, V. H. Knozinger, Metal Clusters in Catalysis, Elsevier, Amsterdam, 1986.
10.1016/S0167-2991(08)65380-X Google Scholar
- 2G. L. Geoffroy, M. S. Wrighton in Organometallic Photochemistry, Academic Press, New York, 1979.
- 3
- 3aA. E. Stiegman, D. R. Tyler, Coord. Chem. Rev. 1985, 63, 217–240;
- 3bT. J. Meyer, J. V. Caspar, Chem. Rev. 1985, 85, 187–218;
- 3cP. C. Ford, J. Organomet. Chem. 1990, 383, 339–356;
- 3dT. E. Bitterwolf, Coord. Chem. Rev. 2001, 211, 235–254.
- 4
- 4aD. Fiedler, D. H. Leung, R. G. Bergman, K. N. Raymond, Acc. Chem. Res. 2005, 38, 349–358;
- 4bM. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. 2009, 121, 3470–3490; Angew. Chem. Int. Ed. 2009, 48, 3418–3438;
- 4cJ. Meeuwissen, J. N. H. Reek, Nat. Chem. 2010, 2, 615–621;
- 4dT. Murase, M. Fujita, Chem. Rec. 2010, 10, 342–347;
- 4eM. J. Wiester, P. A. Ulmann, C. A. Mirkin, Angew. Chem. 2011, 123, 118–142; Angew. Chem. Int. Ed. 2011, 50, 114–137.
- 5
- 5aD. Fiedler, R. G. Bergman, K. N. Raymond, Angew. Chem. 2006, 118, 759–762; Angew. Chem. Int. Ed. 2006, 45, 745–748;
- 5bM. Kuil, T. Soltner, P. W. N. M. van Leeuwen, J. N. H. Reek, J. Am. Chem. Soc. 2006, 128, 11344–11345;
- 5cS. J. Lee, S.-H. Cho, K. L. Mulfort, D. M. Tiede, J. T. Hupp, S. T. Nguyen, J. Am. Chem. Soc. 2008, 130, 16828–16829;
- 5dM. A. Sarmentero, H. Fernamdez-Perez, E. Zuidema, C. Bo, A. Vidal-Ferran, P. Ballester, Angew. Chem. 2010, 122, 7651–7654;
10.1002/ange.201003026 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7489–7492;
- 5eA. Cavarzan, A. Scarso, P. Sgarbossa, G. Strukul, J. N. H. Reek, J. Am. Chem. Soc. 2011, 133, 2848–2851;
- 5fC. J. Brown, G. M. Miller, M. W. Johnson, R. G. Bergman, K. N. Raymond, J. Am. Chem. Soc. 2011, 133, 11964–11966;
- 5gT. Gadzikwa, R. Bellini, H. L. Dekker, J. N. H. Reek, J. Am. Chem. Soc. 2012, 134, 2860–2863.
- 6S. Horiuchi, T. Murase, M. Fujita, J. Am. Chem. Soc. 2011, 133, 12445–12447.
- 7Some research groups reported trapping and stabilization of reaction intermediates within molecular hosts, for example:
- 7aD. J. Cram, M. E. Tanner, R. Thomas, Angew. Chem. 1991, 103, 1048–1051; Angew. Chem. Int. Ed. Engl. 1991, 30, 1024–1027;
- 7bR. Warmuth, Angew. Chem. 1997, 109, 1406–1409;
10.1002/ange.19971091234 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1347–1350;
- 7cR. Warmuth, M. A. Marvel, Angew. Chem. 2000, 112, 1168–1171;
10.1002/(SICI)1521-3757(20000317)112:6<1168::AID-ANGE1168>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1117–1119;10.1002/(SICI)1521-3773(20000317)39:6<1117::AID-ANIE1117>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 7dM. Kawano, Y. Kobayashi, T. Ozeki, M. Fujita, J. Am. Chem. Soc. 2006, 128, 6558–6559;
- 7eT. Iwasawa, R. J. Hooley, J. Rebek, Jr., Science 2007, 317, 493–496.
- 8[(η5-dienyl)Ru(CO)2]2 exists as a mixture of four isomers in solution, see:
- 8aT. E. Bitterwolf, Coord. Chem. Rev. 2000, 206–207, 419–450;
- 8bT. Jaworska, W. Macyk, Z. Stasicka, Struct. Bonding (Berlin) 2004, 106, 153–172.
- 9For a 13C NMR study, see: O. A. Gansow, A. R. Burke, W. D. Vernon, J. Am. Chem. Soc. 1976, 98, 5817–5826.
- 10Host–guest charge-transfer absorption was observed at ca. 600 nm (Figure S5 in the Supporting Information).
- 11M. Egli, S. Sarkhel, Acc. Chem. Res. 2007, 40, 197–205.
- 12UV irradiation over longer periods (>2 h) reduced the yield because of product decomposition. In the absence of alkynes, encapsulated 2 readily decomposed under UV irradiation and any intermediates were not observed, even within the cage. In a control experiment, free 2 immediately decomposed upon irradiation, even in the presence of alkyne 3 a.
- 13Knox and co-workers reported diruthenacyclopentenone analogs by a photoinduced radical reaction, see: A. F. Dyke, S. A. R. Knox, P. J. Naish, G. E. Taylor, J. Chem. Soc. Dalton Trans. 1982, 1297–1307.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.