Enantioselective Total Synthesis of the Reported Structures of (−)-9-epi-Presilphiperfolan-1-ol and (−)-Presilphiperfolan-1-ol: Structural Confirmation and Reassignment and Biosynthetic Insights†
Correction(s) for this article
-
Berichtigung: Enantioselective Total Synthesis of the Reported Structures of (−)-9-epi-Presilphiperfolan-1-ol and (−)-Presilphiperfolan-1-ol: Structural Confirmation and Reassignment and Biosynthetic Insights
- Volume 125Issue 8Angewandte Chemie
- pages: 2201-2201
- First Published online: February 13, 2013
Allen Y. Hong
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)
Search for more papers by this authorCorresponding Author
Prof. Brian M. Stoltz
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)Search for more papers by this authorAllen Y. Hong
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)
Search for more papers by this authorCorresponding Author
Prof. Brian M. Stoltz
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)
Warren and Katharine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 E. California Blvd, MC 101-20, Pasadena, CA 91125 (USA)Search for more papers by this authorThe authors thank NIH-NIGMS (R01GM080269-01), Roche, Abbott Laboratories, Amgen, Boehringer Ingelheim, the Gordon and Betty Moore Foundation, and Caltech for awards and financial support. Prof. Suzana G. Leitão (Universidade Federale do Rio de Janeiro) generously provided NMR spectra for natural 2. Dr. Lawrence Henling is gratefully acknowledged for X-ray crystallographic structure determination. The Bruker KAPPA APEXII X-ray diffractometer used in this study was purchased through an NSF CRIF:MU award to Caltech (CHE-0639094). Prof. Sarah Reisman, Dr. Scott Virgil, Dr. Douglas C. Behenna, Robert J. Ely and Fang Gao (Boston College), and Jessica Y. Wu (Harvard University) are acknowledged for helpful discussions and suggestions. Dr. David VanderVelde and Dr. Scott Ross are acknowledged for NMR assistance.
Graphical Abstract
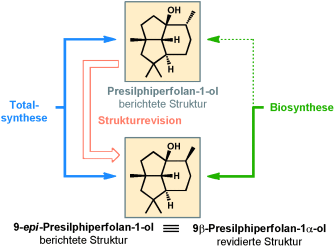
Wenn epi doch nicht epi ist! Die erste Totalsynthese der beschriebenen Strukturen von 9-epi-Presilphiperfolan-1-ol und Presilphiperfolan-1-ol wurde abgeschlossen. Schlüsselschritte sind die katalytische asymmetrische Alkylierung eines neuartigen Dien-Elektrophils mit anschließender C2-Ringkontraktion und eine intramolekulare Diels-Alder-Cycloaddition zur Bildung des dichten tricyclischen Kerns. Die Synthesestudie führt zur Strukturrevision des Presilphiperfolan-1-ols (siehe Schema).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201205276_sm_miscellaneous_information.pdf18.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1F. Bohlmann, J. Jakupovic, Phytochemistry 1980, 19, 259–265.
- 2F. Bohlmann, C. Zdero, J. Jakupovic, H. Robinson, R. M. King, Phytochemistry 1981, 20, 2239–2244.
- 3For representative investigations of presilphiperfolane biosynthesis by isotopic incorporation studies, see the following and references therein:
- 3aJ. R. Hanson, Pure Appl. Chem. 1981, 53, 1155–1162;
- 3bC.-M. Wang, R. Hopson, X. Lin, D. E. Cane, J. Am. Chem. Soc. 2009, 131, 8360–8361.
- 4For reports describing the rearrangement of presilphiperfolanols to related sesquiterpene natural products, see:
- 4aR. M. Coates, Z. Ho, M. Klobus, S. R. Wilson, J. Am. Chem. Soc. 1996, 118, 9249–9254;
- 4bP. Weyerstahl, H. Marschall, I. Seelmann, J. Jakupovic, Eur. J. Org. Chem. 1998, 1205–1212;
10.1002/(SICI)1099-0690(199806)1998:6<1205::AID-EJOC1205>3.0.CO;2-K CAS Web of Science® Google Scholar
- 4cC. E. Davis, B. C. Duffy, R. M. Coates, J. Org. Chem. 2003, 68, 6935–6943.
- 5
- 5aS. Melching, W. A. König, Phytochemistry 1999, 51, 517–523;
- 5b“Isolierung, Strukturaufklärung und stereochemische Untersuchungen neuer sesquiterpenoider Verbindungen aus vier Chemotypen des Lebermooses Conocephalum conicum”: S. Melching, Ph.D. Thesis, Universität Hamburg, April 1999.
- 6
- 6aS. C. Pinto, G. G. Leitão, H. R. Bizzo, N. Martinez, E. Dellacassa, F. M. dos Santos, Jr., F. L. P. Costa, M. B. de Amorim, S. G. Leitão, Tetrahedron Lett. 2009, 50, 4785–4787;
- 6bP. Joseph-Nathan, S. G. Leitão, S. C. Pinto, G. G. Leitão, H. R. Bizzo, F. L. P. Costa, M. B. de Amorim, N. Martinez, E. Dellacassa, A. Hernández-Barragán, N. Pérez-Hernández, Tetrahedron Lett. 2010, 51, 1963–1965.
- 7
- 7aP. Weyerstahl in Newer Trends in Essential Oils and Flavours (Eds.: ), Tata McGraw-Hill, New Delhi, 1993, pp. 24–41;
- 7bJ. A. Marco, J. F. Sanz-Cervera, M. D. Morante, V. Garcia-Lliso, J. Vallès-Xirau, J. Jakupovic, Phytochemistry 1996, 41, 837–844.
- 8For computational studies exploring the presilphiperfolanol biosynthetic pathway, see:
- 8aS. C. Wang, D. J. Tantillo, Org. Lett. 2008, 10, 4827–4830;
- 8bJ. E. Barquera-Lozada, G. Cuevas, J. Org. Chem. 2011, 76, 1572–1577.
- 9For studies describing presilphiperfolane biosynthetic gene clusters, see: C. Pinedo, C.-M. Wang, J.-M. Pradier, B. Dalmais, M. Choquer, P. Le Pêcheur, G. Morgant, I. G. Collado, D. E. Cane, M. Viaud, ACS Chem. Biol. 2008, 3, 791–801.
- 10S. C. Pinto, G. G. Leitão, D. R. de Oliveira, H. R. Bizzo, D. F. Ramos, T. S. Coelho, P. E. A. Silva, M. C. S. Lourenço, S. G. Leitão, Nat. Prod. Commun. 2009, 4, 1675–1678.
- 11A. González-Coloma, F. Valencia, N. Martín, J. F. Hoffmann, L. Hutter, J. A. Marco, M. Reina, J. Chem. Ecol. 2002, 28, 117–129.
- 12
- 12aI. G. Collado, J. Aleu, A. J. Macías-Sánchez, R. Hernández-Galán, J. Chem. Ecol. 1994, 20, 2631–2644;
- 12bI. G. Collado, J. Aleu, A. J. Macías-Sánchez, R. Hernández-Galán, J. Nat. Prod. 1994, 57, 738–746.
- 13Presilphiperfolanol-derived natural products with oxidized core structures have been discovered. See:
- 13aF. Bohlmann, J. Ziesche, R. K. Gupta, Phytochemistry 1982, 21, 1331–1334;
- 13bA. H. Mericli, F. Mericli, J. Jakupovic, F. Bohlmann, X. A. Dominguez, H. S. Vega, Phytochemistry 1989, 28, 1149–1153;
- 13cJ.-L. Yang, L.-L. Liu, Y.-P. Shi, Tetrahedron Lett. 2009, 50, 6315–6317.
- 14To our knowledge, the rearrangement of β-caryophyllene or β-caryophyllene derivatives in biomimetic reactions has not produced any of the presilphiperfolanol natural products. For representative studies, see the following and references therein:
- 14aL. Fitjer, A. Malich, C. Paschke, S. Kluge, R. Gerke, B. Rissom, J. Weiser, M. Noltemeyer, J. Am. Chem. Soc. 1995, 117, 9180–9189;
- 14bS. Shankar, R. M. Coates, J. Org. Chem. 1998, 63, 9177–9182.
- 15The rearrangement of isocaryophyllene with Brønsted or Lewis acids has yielded unnatural isomeric presilphiperfolanol derivatives. For representative studies, see the following and references therein:
- 15aK. Gollnick, G. Schade, A. F. Cameron, C. Hannaway, J. S. Roberts, J. M. Robertson, J. Chem. Soc. Chem. Commun. 1970, 248–249;
- 15bT. M. Khomenko, I. Y. Bagryanskaya, Y. V. Gatilov, D. V. Korchagina, V. P. Gatilova, Z. V. Dubovenko, V. A. Barkhash, Zh. Org. Khim. 1985, 21, 677–678. Russ. J. Org. Chem. (Engl. Transl.) 1985, 21, 614–615;
- 15calso see ref. [12].
- 16P. Weyerstahl, H. Marschall, M. Schulze, I. Schwope, Liebigs Ann. 1996, 799–807.
- 17“Carbocycle Construction in Terpenoid Synthesis. The Total Synthesis of (±)-Sarcodonin G and (±)-1-Epi-9-Norpresilphiperfolan-9-one”: M. W. Gilbert, Ph.D. Thesis, University of British Columbia, June 2002.
- 18
- 18aP. Weyerstahl, H. Marschall, M. Schröder, H.-C. Wahlburg, V. K. Kaul, Flavour Fragrance J. 1997, 12, 315–325;
10.1002/(SICI)1099-1026(199709/10)12:5<315::AID-FFJ662>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 18bC. Menut, G. Lamaty, P. Weyerstahl, H. Marschall, I. Seelmann, P. H. Amvam Zollo, Flavour Fragrance J. 1997, 12, 415–421.
- 19E. Osawa, K. Aigami, N. Takaishi, Y. Inamoto, Y. Fujikura, Z. Majerski, P. v. R. Schleyer, E. M. Engler, M. Farcasiu, J. Am. Chem. Soc. 1977, 99, 5361–5373.
- 20
- 20aA. Y. Hong, M. R. Krout, T. Jensen, N. B. Bennett, A. M. Harned, B. M. Stoltz, Angew. Chem. 2011, 123, 2808–2812; Angew. Chem. Int. Ed. 2011, 50, 2756–2760;
- 20bA. Y. Hong, N. B. Bennett, M. R. Krout, T. Jensen, A. M. Harned, B. M. Stoltz, Tetrahedron 2011, 67, 10234–10248;
- 20cN. B. Bennett, A. Y. Hong, B. M. Stoltz, Org. Biomol. Chem. 2012, 10, 56–59.
- 21Carbamate 15 was prepared from commercial 2-methyl-2-vinyloxirane by modification of reported procedures. See:
- 21aY. Satake, T. Nishikawa, T. Hiramatsu, H. Araki, M. Isobe, Synthesis 2010, 1992–1998;
- 21bS. T. Heller, R. Sarpong, Org. Lett. 2010, 12, 4572–4575.
- 22Substituted allylic carbamates have been used by Trost to achieve O-acylation of enolates under different reaction conditions. See: B. M. Trost, J. Xu, J. Org. Chem. 2007, 72, 9372–9375.
- 23L. N. Mander, S. P. Sethi, Tetrahedron Lett. 1983, 24, 5425–5428.
- 24
- 24aD. C. Behenna, B. M. Stoltz, J. Am. Chem. Soc. 2004, 126, 15044–15045;
- 24bJ. T. Mohr, D. C. Behenna, A. M. Harned, B. M. Stoltz, Angew. Chem. 2005, 117, 7084–7087;
10.1002/ange.200502018 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6924–6927;
- 24cD. C. Behenna, J. T. Mohr, N. H. Sherden, S. C. Marinescu, A. M. Harned, K. Tani, M. Seto, S. Ma, Z. Novák, M. R. Krout, R. M. McFadden, J. L. Roizen, J. A. Enquist, Jr., D. E. White, S. R. Levine, K. V. Petrova, A. Iwashita, S. C. Virgil, B. M. Stoltz, Chem. Eur. J. 2011, 17, 14199–14223.
- 25
- 25aP. von Matt, A. Pfaltz, Angew. Chem. 1993, 105, 614–615; Angew. Chem. Int. Ed. Engl. 1993, 32, 566–568;
- 25bJ. Sprinz, G. Helmchen, Tetrahedron Lett. 1993, 34, 1769–1772;
- 25cG. J. Dawson, C. G. Frost, J. M. J. Williams, S. J. Coote, Tetrahedron Lett. 1993, 34, 3149–3150.
- 26
- 26aK. Tani, D. C. Behenna, R. M. McFadden, B. M. Stoltz, Org. Lett. 2007, 9, 2529–2531;
- 26bM. R. Krout, J. T. Mohr, B. M. Stoltz, Org. Synth. 2009, 86, 181–193.
- 27For examples of the asymmetric alkylation of vinylogous esters or thioesters in the synthesis of natural products from our laboratory, see:
- 27aD. E. White, I. C. Stewart, R. H. Grubbs, B. M. Stoltz, J. Am. Chem. Soc. 2008, 130, 810–811;
- 27bS. R. Levine, M. R. Krout, B. M. Stoltz, Org. Lett. 2009, 11, 289–292;
- 27cK. V. Petrova, J. T. Mohr, B. M. Stoltz, Org. Lett. 2009, 11, 293–295.
- 28For a related example of the asymmetric alkylation of vinylogous thioesters, see: B. M. Trost, R. N. Bream, J. Xu, Angew. Chem. 2006, 118, 3181–3184;
10.1002/ange.200504421 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3109–3112.
- 29For selected IMDA reviews, see:
- 29aB. R. Bear, S. M. Sparks, K. J. Shea, Angew. Chem. 2001, 113, 864–894;
10.1002/1521-3757(20010302)113:5<864::AID-ANGE864>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 820–849;10.1002/1521-3773(20010302)40:5<820::AID-ANIE820>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 29bK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. 2002, 114, 1742–1773;
10.1002/1521-3757(20020517)114:10<1742::AID-ANGE1742>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1668–1698;10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 29cK.-i. Takao, R. Munakata, K.-i. Tadano, Chem. Rev. 2005, 105, 4779–4807;
- 29dM. Juhl, D. Tanner, Chem. Soc. Rev. 2009, 38, 2983–2992.
- 30A related IMDA cycloaddition for the construction of an analogous tricyclic system with an activated alkyne has been reported. See: L. Evanno, A. Deville, B. Bodo, B. Nay, Tetrahedron Lett. 2007, 48, 4331–4333.
- 31Relevant IMDA studies performed by Jung and Corey demonstrated that the diastereoselectivity of normal-demand Type I IMDA reactions can be reversed by substituting enone dienophiles with their corresponding sterically demanding cyclic ketal derivatives. See:
- 31aM. E. Jung, K. M. Halweg, Tetrahedron Lett. 1981, 22, 3929–3932;
- 31bE. J. Corey, P. A. Magriotis, J. Am. Chem. Soc. 1987, 109, 287–289.
- 32E. H. Krenske, E. W. Perry, S. V. Jerome, T. J. Maimone, P. S. Baran, K. N. Houk, Org. Lett. 2012, 14, 3016–3019.
- 33Our IMDA substrate does not fit neatly into the Type I or Type II IMDA classification, making prediction of the reaction outcome less straightforward.
- 34aM. Satoh, Y. Nomoto, N. Miyaura, A. Suzuki, Tetrahedron Lett. 1989, 30, 3789–3792;
- 34bJ. Y. Wu, B. Moreau, T. Ritter, J. Am. Chem. Soc. 2009, 131, 12915–12917;
- 34cR. J. Ely, J. P. Morken, J. Am. Chem. Soc. 2010, 132, 2534–2535;
- 34dR. J. Ely, J. P. Morken, Org. Synth. 2011, 88, 342–352.
- 35See Supporting Information for additional details and experimental procedures.
- 36C. A. Luchaco-Cullis, H. Mizutani, K. E. Murphy, A. H. Hoveyda, Angew. Chem. 2001, 113, 1504–1508;
10.1002/1521-3757(20010417)113:8<1504::AID-ANGE1504>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1456–1460.10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 37R. W. Murray, M. Singh, Org. Synth. 1997, 74, 91–96.
- 38CCDC 889569 (1) and 889570 (21 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 39R. H. Crabtree, M. W. Davis, J. Org. Chem. 1986, 51, 2655–2661.
- 40N. Kotoku, Y. Sumii, M. Kobayashi, Org. Lett. 2011, 13, 3514–3517.
- 41Unfortunately, attempts to obtain samples or spectra of naturally isolated presilphiperfolan-1-ol (1) were unsuccessful.
- 42An X-ray crystal structure for a synthetic compound with the same structure as our synthetic 1 (prepared from isocaryophyllene) was reported by Khomenko and co-workers (see Ref. [15b]). Our NMR data for synthetic 1 matches the spectral data for Khomenko’s synthetic 1 but not the spectral data for König’s reported natural 1.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.