Actuation of Asymmetric Cyclopropanation Catalysts: Reversible Single-Crystal to Single-Crystal Reduction of Metal–Organic Frameworks†
Joseph M. Falkowski
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorCheng Wang
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorSophie Liu
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorCorresponding Author
Prof. Wenbin Lin
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.htmlSearch for more papers by this authorJoseph M. Falkowski
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorCheng Wang
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorSophie Liu
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorCorresponding Author
Prof. Wenbin Lin
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA) http://www.chem.unc.edu/people/faculty/linw/wlindex.htmlSearch for more papers by this authorWe thank the NSF (CHE-0809776) for financial support, and Kathryn DeKrafft and Rachel Huxford for experimental help. J.M.F. is supported by a DOE Office of Science graduate fellowship under the DOE contract number DE-AC05-06OR23100. C.W. acknowledges the UNC Department of Chemistry for an Ernest L. Eliel Fellowship. S.L. is supported by a UNC William W. and Ida W. Taylor Fellowship.
Graphical Abstract
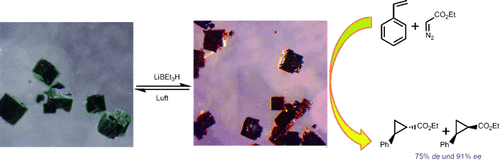
Nur reduziert aktiv: Chirale Metall-organische Gerüste (CMOFs) mit redoxaktiven Ruthenium-Salen-Bausteinen gehen eine reversible Reduktion und Oxidation im Einkristall ein. Nicht katalytisch aktive RuIII-CMOFs (grün) wurden zu RuII-CMOFs (rot) reduziert, die hoch diastereo- und enantioselektive Cyclopropanierungen von Alkenen vermitteln.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104086_sm_miscellaneous_information.pdf3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Dinca, J. R. Long, Angew. Chem. 2008, 120, 6870;
10.1002/ange.200801163 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6766;
- 1bJ. L. Rowsell, O. M. Yaghi, Angew. Chem. 2005, 117, 4748;
10.1002/ange.200462786 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4670;
- 1cB. Kesanli, Y. Cui, M. R. Smith, E. W. Bittner, B. C. Bockrath, W. Lin, Angew. Chem. 2005, 117, 74;
10.1002/ange.200461214 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 72;
- 1dW. Yang. A. Greenaway, X. Lin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, P. Hubberstey, S. Kitagawa, N. R. Champness, M. Schröder, J. Am. Chem. Soc. 2010, 132, 14457.
- 2
- 2aM. D. Allendorf, R. J. Houk, L. Andruszkiewicz, A. A. Talin, J. Pikarsky, A. Choudhury, K. A. Gall, P. J. Hesketh, J. Am. Chem. Soc. 2008, 130, 14404;
- 2bB. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui, G. Qian, Angew. Chem. 2009, 121, 508; Angew. Chem. Int. Ed. 2009, 48, 500;
- 2cS. Pramanik, C. Zheng, X. Zhang, T. J. Emge, J. Li, J. Am. Chem. Soc. 2011, 133, 4153–4155;
- 2dZ. Xie, L. Ma, K. E. deKrafft, A. Jin, W. Lin, J. Am. Chem. Soc. 2010, 132, 922.
- 3
- 3aB. Kesanli, W. Lin, Coord. Chem. Rev. 2003, 246, 305;
- 3bJ. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450;
- 3cL. Ma, C. Abney, W. Lin, Chem. Soc. Rev. 2009, 38, 1248;
- 3dG. Nickerl, A. Henschel, R. Grunker R, K. Gedrich, S. Kaskel, Chem. Ing. Tech. 2011, 83, 90.
- 4
- 4aK. E. deKrafft, Z. Xie, G. Cao, S. Tran, L. Ma, O. Z. Zhou, W. Lin, Angew. Chem. 2009, 121, 10085;
10.1002/ange.200904958 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9901;
- 4bW. Lin, W. J. Rieter, K. M. Taylor, Angew. Chem. 2009, 121, 660; Angew. Chem. Int. Ed. 2009, 48, 650.
- 5
- 5aW. J. Rieter, K. M. Pott, K. M. L. Taylor, W. Lin, J. Am. Chem. Soc. 2008, 130, 11584;
- 5bP. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur, R. Gref, Nat. Mater. 2010, 9, 172;
- 5cK. M. L. Taylor-Pashow, J. Della Rocca, Z. Xie, S. Tran, W. Lin, J. Am. Chem. Soc. 2009, 131, 14261.
- 6L. Ma, C.-D. Wu, M. M. Wanderley, W. Lin, Angew. Chem. 2010, 122, 8420; Angew. Chem. Int. Ed. 2010, 49, 8244.
- 7
- 7aA. Hu, H. L. Ngo, W. Lin, J. Am. Chem. Soc. 2003, 125, 11490;
- 7bA. Hu, H. L. Ngo, W. Lin, Angew. Chem. 2003, 115, 6182; Angew. Chem. Int. Ed. 2003, 42, 6000.
- 8
- 8aT. P. Yoon, E. N. Jacobsen, Science 2003, 299, 1691;
- 8bM. Palucki, N. S. Finney, P. J. Pospisil, M. L. Guler, T. Ishida, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 948;
- 8cW. Zhang, J. L. Loebach, S. R. Wilson, E. N. Jacobsen, J. Am. Chem. Soc. 1990, 112, 2801.
- 9
- 9aM. Oh, C. A. Mirkin, Angew. Chem. 2006, 118, 5618;
10.1002/ange.200601918 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5492;
- 9bY. M. Jeon, J. Heo, C. A. Mirkin, J. Am. Chem. Soc. 2007, 129, 7480;
- 9cS. Jung, M. Oh, Angew. Chem. 2008, 120, 2079; Angew. Chem. Int. Ed. 2008, 47, 2049;
- 9dR. Kitaura, G. Onoyama, H. Sakamoto, R. Matsuda, S. Noro, S. Kitagawa, Angew. Chem. 2004, 116, 2738;
10.1002/ange.200352596 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2684;
- 9eB. Chen, X. Zhao, A. Putkham, K. Hong, E. B. Lobkovsky, E. J. Hurtado, A. J. Fletcher, K. M. Thomas, J. Am. Chem. Soc. 2008, 130, 6411.
- 10G. Yuan, C. Zhu, W. Xuan, Y. Cui, Chemistry 2009, 15, 6428.
- 11
- 11aS.-H. Cho, B. Ma, S. T. Nguyen, J. T. Hupp, T. E. Albrecht-Schmitt, Chem. Commun. 2006, 2563;
- 11bF. Song, C. Wang, J. M. Falkowski, L. Ma, W. Lin, J. Am. Chem. Soc. 2010, 132, 15390–15398; F. Song, C. Wang, W. Lin, Chem. Commun. 2011, 47, 5256–8258.
- 12
- 12aH. J. Choi, M. P. Suh, J. Am. Chem. Soc. 2004, 126, 15844;
- 12bM. P. Suh, Y. E. Cheon, Aust. J. Chem. 2006, 59, 605;
- 12cY.-G. Huang, B. Mu, P. M. Schoenecker, C. G. Carson, J. R. Karra, Y. Cai, K. S. Walton, Angew. Chem. 2011, 123, 456; Angew. Chem. Int. Ed. 2011, 50, 436.
- 13M. Meilikhov, K. Yusenko, A. Torrisi, B. Jee, C. Mellot-Draznieks, A. Pöppl, R. A. Fischer, Angew. Chem. 2010, 122, 6348;
10.1002/ange.200907126 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6212.
- 14The determination of the unit cell and data collections for crystals of [Ru(L-H2)(Py)2]Cl, 1, 1 R, 2, and 2 R were performed on a Bruker SMART APEX II diffractometer. Crystallographic data for [Ru(L-H2)(Py)2]Cl: Monoclinic, space group C2, a=19.703(1), b=25.895(2), c=18.631(2) Å, β=90.381(4)°, V=9505.5(10) Å3, Z=8, ρcalcd=1.321 g cm−3, R1(I>2 σ(I))=0.0718 , wR2(I>2 σ(I))=0.174. Crystallographic data for 1: Trigonal, space group R32, a=29.750 (2), c=72.886(7) Å, V=55 865(7) Å3, Z=6, ρcalcd=0.884 g cm−3, R1(I>2 σ(I))=0.177 , wR2(I>2 σ(I))=0.401. Crystallographic data for 2: Trigonal, space group R3, a=29.681 (1), c=72.690(2) Å, V=55 457(2) Å3, Z=3, ρcalcd=0.445 g cm−3, R1(I>2 σ(I))=0.138 , wR2(I>2 σ(I))=0.337. Crystallographic data for 1 R: Trigonal, space group R32, a=29.798 (1), c=73.027(2) Å, V=56 153(2) Å3, Z=6, ρcalcd=0.879 g cm−3, R1(I>2 σ(I))=0.163 , wR2(I>2 σ(I))=0.376. Crystallographic data for 2 R: Trigonal, space group R3, a=29.724 (1), c=72.861(2) Å, V=55 750(2) Å3, Z=3, ρcalcd=0.443 g cm−3, R1(I>2 σ(I))=0.171 , wR2(I>2 σ(I))=0.263. CCDC 816123, 816124, 816125, 816126, and 816127 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15The solvent-dependent catenation behavior observed here is different from the CMOFs built from manganese/salen struts (see Ref. [11b]) and probably a result of different solubility of the CMOFs in these solvents.
- 16L. Ma, J. M. Falkowski, C. Abney, W. Lin, Nat. Chem. 2010, 2, 838.
- 17M. Muthukumar, P. Viswanathamurthi, K. Natarajan, Spectrochim. Acta Part A 2008, 70, 1222.
- 18J. A. Miller, W. Jin, S. T. Nguyen, Angew. Chem. 2002, 114, 3077;
10.1002/1521-3757(20020816)114:16<3077::AID-ANGE3077>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2953.10.1002/1521-3773(20020816)41:16<2953::AID-ANIE2953>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 19M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe, O. M. Yaghi, Science 2002, 295, 469.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.