Developing Synthetic Approaches with Non-Innocent Metalloligands: Easy Access to IrI/Pd0 and IrI/Pd0/IrI Cores†
Corresponding Author
Dr. Cristina Tejel
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)Search for more papers by this authorLaura Asensio
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorDr. M. Pilar del Río
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorDr. Bas de Bruin
Van't Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam (UvA), Science Park 904, 1098 XH Amsterdam (The Netherlands)
Search for more papers by this authorDr. José A. López
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorProf. Dr. Miguel A. Ciriano
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorCorresponding Author
Dr. Cristina Tejel
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)Search for more papers by this authorLaura Asensio
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorDr. M. Pilar del Río
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorDr. Bas de Bruin
Van't Hoff Institute for Molecular Sciences (HIMS), University of Amsterdam (UvA), Science Park 904, 1098 XH Amsterdam (The Netherlands)
Search for more papers by this authorDr. José A. López
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorProf. Dr. Miguel A. Ciriano
Instituto de Síntesis Química y Catálisis Homogénea (ISQCH), CSIC—Universidad de Zaragoza, Pedro Cerbuna 12, 50009-Zaragoza (Spain)
Search for more papers by this authorThe generous financial support from MICINN/FEDER (Project CTQ2008-03860) and Gobierno de Aragón (GA, Project: PI 155/08) is gratefully acknowledged. L.A. and P.R. thank MICINN/FEDER for a fellowship and a post-doctoral contract, respectively.
Graphical Abstract
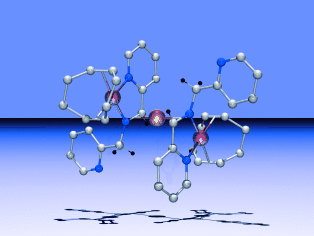
Schuldig der Anklage, so lautet das Urteil für den anionischen Iridium-Komplex [Ir(bpa−2 H)(cod)]− in Reaktionen mit Palladium(II)-Verbindungen. Der Transfer von zwei Elektronen vom Ir-Komplex zu Pd ermöglicht die einfache Herstellung von zwei- und dreikernigen π-Imin-koordinierten Pd0-Verbindungen wie [{Ir(PyCH2NCHPy)(cod)}2Pd] (siehe Bild; C weiß, Ir rot, N blau, Pd gelb). bpa−2 H: doppelt deprotoniertes N,N-Bis(2-picolyl)amin (bpa); cod: 1,5-Cyclooctadien.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201104045_sm_miscellaneous_information.pdf4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Examples of general reviews:
- 1aS. Sproules, K. Wieghardt, Coord. Chem. Rev. 2010, 254, 1358–1382;
- 1bA. B. P. Lever, Coord. Chem. Rev. 2010, 254, 1397–1405;
- 1cW. Kaim, B. Schwederski, Coord. Chem. Rev. 2010, 254, 1580–1588;
- 1dP. Deplano, L. Pilia, D. Espa, M. L. Mercuri, A. Serpe, Coord. Chem. Rev. 2010, 254, 1434–1447;
- 1eJ. L. Boyer, J. Rochford, M.-K. Tsai, J. T. Muckerman, E. Fujita, Coord. Chem. Rev. 2010, 254, 309–330;
- 1fK. Ray, T. Petrenko, K. Wieghardt, F. Neese, Dalton Trans. 2007, 1552–1566;
- 1gD. G. H. Hetterscheid, H. Grützmacher, A. J. J. Koekkoek, B. de Bruin, Prog. Inorg. Chem. 2007, 55, 247–253;
- 1hB. de Bruin, D. G. H. Hetterscheid, Eur. J. Inorg. Chem. 2007, 211–230;
- 1iE. Evangelio, D. Ruiz-Molina, Eur. J. Inorg. Chem. 2005, 2957–2971;
- 1jM. D. Ward, J. A. McCleverty, J. Chem. Soc. Dalton Trans. 2002, 275–288;
- 1kC. G. Pierpont, Coord. Chem. Rev. 2001, 216–217, 99–125.
- 2Recent leading references involving noninnocent ligands:
- 2aM. M. Khusniyarov, E. Bill, T. Weyhermüller, E. Bothe, K. Wieghardt, Angew. Chem. 2011, 123, 1690–1693; Angew. Chem. Int. Ed. 2011, 50, 1652–1655;
- 2bW. I. Dzik, X. P. Zhang, B. de Bruin, Inorg. Chem. 2011, DOI: ;
- 2cW. I. Dzik, S. E. Calvo, J. N. H. Reek, M. Lutz, M. A. Ciriano, C. Tejel, D. G. H. Hetterscheid, B. de Bruin, Organometallics 2011, 30, 372–374;
- 2dH. Lu, W. I. Dzik, X. Xu, L. Wojtas, B. de Bruin, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 8518–8521;
- 2eY. M. Badiei, M. A. Siegler, D. P. Goldberg, J. Am. Chem. Soc. 2011, 133, 1274–1277;
- 2fA. I. Olivos Suarez, H. Jiang, X. P. Zhang, B. de Bruin, Dalton Trans. 2011, 40, 5697–5705;
- 2gN. D. Paul, T. Krämer, J. E. McGrady, S. Goswami, Chem. Commun. 2010, 46, 7124–7126;
- 2hA. L. Smith, K. I. Hardcastle, J. D. Soper, J. Am. Chem. Soc. 2010, 132, 14358–14360;
- 2iA. Paretzki, R. Pattacini, R. Huebner, P. Braunstein, B. Sarkar, Chem. Commun. 2010, 46, 1497–1499;
- 2jC. R. Hess, T. Weyhermüller, E. Bill, K. Wieghardt, Inorg. Chem. 2010, 49, 5686–5700;
- 2kN. L. Wieder, M. Gallagher, P. J. Carroll, D. H. Berry, J. Am. Chem. Soc. 2010, 132, 4107–4109;
- 2lF. F. Puschmann, J. Harmer, D. Stein, H. Ruegger, B. de Bruin, H. Grützmacher, Angew. Chem. 2010, 122, 395–399;
10.1002/ange.200903201 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 385–389;
- 2mM. R. Ringenberg, M. J. Nilges, T. B. Rauchfuss, S. R. Wilson, Organometallics 2010, 29, 1956–1965;
- 2nF. F. Puschmann, H. Grützmacher, B. de Bruin, J. Am. Chem. Soc. 2010, 132, 73–75;
- 2oA. M. Tondreau, C. Milsmann, A. D. Patrick, H. M. Hoyt, E. Lobkovsky, K. Wieghardt, P. J. Chirik, J. Am. Chem. Soc. 2010, 132, 15046–15059;
- 2pE. Evangelio, M.-L. Bonnet, M. Cabañas, M. Nakano, J.-P. Sutter, A. Dei, V. Robert, D. Ruiz-Molina, Chem. Eur. J. 2010, 16, 6666–6677;
- 2qW. I. Dzik, X. Xu, X. P. Zhang, J. N. H. Reek, B. de Bruin, J. Am. Chem. Soc. 2010, 132, 10891–10902;
- 2rA. K. Das, B. Sarkar, C. Duboc, S. Strobel, J. Fiedler, S. Záliš, G. K. Lahiri, W. Kaim, Angew. Chem. 2009, 121, 4306–4309; Angew. Chem. Int. Ed. 2009, 48, 4242–4245;
- 2sC. R. Hess, T. Weyhermüller, E. Bill, K. Wieghardt, Angew. Chem. 2009, 121, 3758–3761;
10.1002/ange.200900725 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3703–3706;
- 2tR. G. Hicks, Angew. Chem. 2008, 120, 7503–7505;
10.1002/ange.200802713 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7393–7395.
- 3
- 3aH. Grützmacher, Angew. Chem. 2008, 120, 1838–1842; Angew. Chem. Int. Ed. 2008, 47, 1814–1818;
- 3bP. Chaudhuri, M. Hess, U. Flörke, K. Wieghardt, Angew. Chem. 1998, 110, 2340–2343;
10.1002/(SICI)1521-3757(19980817)110:16<2340::AID-ANGE2340>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2217–2220.10.1002/(SICI)1521-3773(19980904)37:16<2217::AID-ANIE2217>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 4
- 4aW. I. Dzik, J. I. van der Vlugt, J. N. H. Reek, B. de Bruin, Angew. Chem. 2011, 123, 3416–3418; Angew. Chem. Int. Ed. 2011, 50, 3356–3358;
- 4bP. J. Chirik, K. Wieghardt, Science 2010, 327, 794–795.
- 5
- 5aT. Stahl, V. Kasack, W. Kaim, J. Chem. Soc. Perkin Trans. 2 1995, 2127–2131;
- 5bE. Uhlig, Pure Appl. Chem. 1988, 60, 1235–1240;
- 5cG. van Koten, J. T. B. H. Jastrzebski, K. Vrieze, J. Organomet. Chem. 1983, 250, 49–61.
- 6C. C. Lu, E. Bill, T. Weyhermüller, E. Bothe, K. Wieghardt, J. Am. Chem. Soc. 2008, 130, 3181–3197.
- 7
- 7aW. Massa, S. Dehghanpour, K. Jahani, Inorg. Chim. Acta 2009, 362, 2872–2878, and references therein;
- 7bS. Dehghanpour, M. Khalaj, A. Mahmoudi, Polyhedron 2009, 28, 1205–1210;
- 7cJ. Kuwabara, D. Takeuchi, K. Osakada, Polyhedron 2009, 28, 2459–2465;
- 7dA. Lavalette, F. Tuna, G. Clarkson, N. W. Alcock, M. J. Hannon, Chem. Commun. 2003, 2666–2667;
- 7eR. Ziessel, L. Douce, A. El-ghayoury, A. Harriman, A. Skoulios, Angew. Chem. 2000, 112, 1549–1553;
10.1002/(SICI)1521-3757(20000417)112:8<1549::AID-ANGE1549>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1489–1493;10.1002/(SICI)1521-3773(20000417)39:8<1489::AID-ANIE1489>3.0.CO;2-2 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 1549–1553.
- 8
- 8aC. Alonso-Moreno, F. Carrillo-Hermosilla, J. Romero-Fernández, A. M. Rodríguez, A. Otero, A. Antiñolo, Adv. Synth. Catal. 2009, 351, 881–890;
- 8bB. Moreau, J. Y. Wu, T. Ritter, Org. Lett. 2009, 11, 337–339;
- 8cJ. D. A. Pelletier, J. Fawcett, K. Singh, G. A. Solan, J. Organomet. Chem. 2008, 693, 2723–2731;
- 8dS. Mori, M. Nambo, L.-C. Chi, J. Bouffard, K. Itami, Org. Lett. 2008, 10, 4609–4612;
- 8eJ. Song, Q. Shen, F. Xu, X. Lu, Tetrahedron 2007, 63, 5148–5153;
- 8fJ. Kuwabara, D. Takeuchi, K. Osakada, Chem. Commun. 2006, 3815–3817.
- 9
- 9aC. C. Lu, T. Weyhermüller, E. Bill, K. Wieghardt, Inorg. Chem. 2009, 48, 6055–6064; Cr complexes:
- 9bC. C. Lu, S. D. George, T. Weyhermüller, E. Bill, E. Bothe, K. Wieghardt, Angew. Chem. 2008, 120, 6484–6487; Angew. Chem. Int. Ed. 2008, 47, 6384–6387; Zn complexes:
- 9cM. van Gastel, C. C. Lu, K. Wieghardt, W. Lubitz, Inorg. Chem. 2009, 48, 2626–2632;
- 9dA. Mondal, T. Weyhermüller, K. Wieghardt, Chem. Commun. 2009, 6098–6100.
- 10
- 10aA. A. Trifonov, I. D. Gudilenkov, J. Larionova, C. Luna, G. K. Fukin, A. V. Cherkasov, A. I. Poddel’sky, N. O. Druzhkov, Organometallics 2009, 28, 6707–6713;
- 10bA. A. Trifonov, E. A. Fedorova, I. A. Borovkov, G. K. Fukin, E. V. Baranov, J. Larionova, N. O. Druzhkov, Organometallics 2007, 26, 2488–2491.
- 11
- 11aA. Malassa, C. Agthe, H. Görls, M. Friedrich, M. Westerhausen, J. Organomet. Chem. 2010, 695, 1641–1650;
- 11bC. Stanciu, M. E. Jones, P. E. Fanwick, M. M. Abu-Omar, J. Am. Chem. Soc. 2007, 129, 12400–12401;
- 11cM. Westerhausen, A. N. Kneifel, I. Lindner, J. Grčić, H. Nöth, Z. Naturforsch. B 2004, 59, 161–166;
- 11dM. Westerhausen, T. Bollwein, N. Makropoulos, S. Schneiderbauer, M. Suter, H. Nöth, P. Mayer, H. Piotrowski, K. Polborn, A. Pfitzner, Eur. J. Inorg. Chem. 2002, 389–404.
- 12
- 12aC. Tejel, M. P. del Río, M. A. Ciriano, E. J. Reijerse, F. Hartl, S. Záliš, D. G. H. Hetterscheid, N. Tsichlis i Spithas, B. de Bruin, Chem. Eur. J. 2009, 15, 11878–11889;
- 12bC. Tejel, M. A. Ciriano, M. P. del Rio, D. G. H. Hetterscheid, N. Tsichlis i Spitas, J. M. M. Smits, B. de Bruin, Chem. Eur. J. 2008, 14, 10932–10936.
- 13C. Tejel, M. A. Ciriano, M. P. del Río, F. J. van den Bruele, D. G. H. Hetterscheid, N. Tsichlis i Spithas, B. de Bruin, J. Am. Chem. Soc. 2008, 130, 5844–5845.
- 14
- 14aA. Brennführer, H. Neumann, M. Beller, Angew. Chem. 2009, 121, 4176–4196;
10.1002/ange.200900013 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4114–4133;
- 14bJ. Tsuji, Palladium Reagents and Catalysts: New Perspectives for the 21st Century, 2nd ed., Wiley, Chichester, 2004.
10.1002/0470021209 Google Scholar
- 15
- 15aN. C. Tomson, L. A. Labios, T. Weyhermüller, J. S. Figueroa, K. Wieghardt, Inorg. Chem. 2011, DOI: ;
- 15bN. Deibel, D. Schweinfurth, R. Huebner, P. Braunstein, B. Sarkar, Dalton Trans. 2011, 40, 431–436;
- 15cD. A. Smith, A. S. Batsanov, K. Costuas, R. Edge, D. C. Apperley, D. Collison, J.-F. Halet, J. A. K. Howard, P. W. Dyer, Angew. Chem. 2010, 122, 7194–7198; Angew. Chem. Int. Ed. 2010, 49, 7040–7044;
- 15dC. Mukherjee, T. Weyhermüller, E. Bothe, P. Chaudhuri, Inorg. Chem. 2008, 47, 11620–11632;
- 15eJ. Zhou, H. Sun, K. Harms, J. Sundermeyer, Z. Anorg. Allg. Chem. 2008, 634, 1517–1521;
- 15fI. J. S. Fairlamb, A. R. Kapdi, A. F. Lee, G. P. McGlacken, F. Weissburger, A. H. M. de Vries, L. Schmieder-van de Vondervoort, Chem. Eur. J. 2006, 12, 8750–8761;
- 15gI. J. S. Fairlamb, C. T. O’Brien, Z. Lin, K. C. Lam, Org. Biomol. Chem. 2006, 4, 1213–1216.
- 16CCDC 821731 (2), 821732 (4) and 821733 (5), contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 17Angles around the palladium center are 126.4(2), 129.7(2), and 103.6(1)° for Cl-Pd-Ct, P-Pd-Ct ,and Cl-Pd-P, respectively with Σ0=359.7(4) (Ct represents the midpoint of the C6N2 bond).
- 18
- 18aW. Li, X. Zhang, A. Meetsma, B. Hessen, Organometallics 2008, 27, 2052–2057;
- 18bC. C. Lu, J. C. Peters, J. Am. Chem. Soc. 2004, 126, 15818–15832;
- 18cG. R. Owen, R. Vilar, A. J. P. White, D. J. Williams, Organometallics 2003, 22, 3025–3027.
- 19S. K. Russell, C. Milsmann, E. Lobkovsky, T. Weyhermüller, P. J. Chirik, Inorg. Chem. 2011, 50, 3159–3169.
- 20S. Kozuch, S. Shaik, A. Jutand, C. Amatore, Chem. Eur. J. 2004, 10, 3072–3080.
- 21Preliminary catalytic studies on the standard Heck reaction (C6H5I+H2CCHCN) with complex 2 as catalyst precursor indicate moderate catalytic activity at 100 °C in toluene.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.