Enantioselective Michael/Mannich Polycyclization Cascade of Indolyl Enones Catalyzed by Quinine-Derived Primary Amines†
Quan Cai
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorChao Zheng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorJun-Wei Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/Search for more papers by this authorQuan Cai
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorChao Zheng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorJun-Wei Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
Search for more papers by this authorCorresponding Author
Prof. Dr. Shu-Li You
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China) http://shuliyou.sioc.ac.cn/Search for more papers by this authorWe thank the National Natural Science Foundation of China (20732006, 20821002, 21025209), the National Basic Research Program of China (973 Program 2010CB833300), and the Chinese Academy of Sciences for generous financial support.
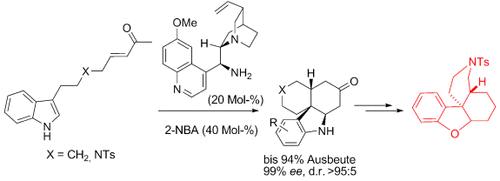
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201103937_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aK. C. Nicolaou, D. J. Edmonds, P. G. Bulger, Angew. Chem. 2006, 118, 7292;
10.1002/ange.200601872 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7134;
- 1bC. J. Chapman, C. G. Frost, Synthesis 2007, 1;
- 1cA. M. Walji, D. W. C. MacMillan, Synlett 2007, 1477;
- 1dP. Melchiorre, M. Marigo, A. Carlone, G. Bartoli, Angew. Chem. 2008, 120, 6232;
10.1002/ange.200705523 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6138;
- 1eA. Mielgo, C. Palomo, Chem. Asian J. 2008, 3, 922;
- 1fH. M. L. Davies, E. J. Sorensen, Chem. Soc. Rev. 2009, 38, 2981;
- 1gK. C. Nicolaou, J. S. Chen, Chem. Soc. Rev. 2009, 38, 2993;
- 1hC. Grondal, M. Jeanty, D. Enders, Nat. Chem. 2010, 2, 167, and references therein.
- 2For selected examples of indolenine syntheses, see:
- 2aN. Kagawa, J. P. Malerich, V. H. Rawal, Org. Lett. 2008, 10, 2381, and references therein;
- 2bK. Eastman, P. S. Baran, Tetrahedron 2009, 65, 3149;
- 2cQ.-F. Wu, H. He, W.-B. Liu, S.-L. You, J. Am. Chem. Soc. 2010, 132, 11418.
- 3Selected examples:
- 3aR. B. Woodward, M. P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker, J. Am. Chem. Soc. 1954, 76, 4749;
- 3bR. B. Woodward, M. P. Cava, W. D. Ollis, A. Hunger, H. U. Daeniker, K. Schenker, Tetrahedron 1963, 19, 247;
- 3cS. P. Marsden, K. M. Depew, S. J. Danishefsky, J. Am. Chem. Soc. 1994, 116, 11143;
- 3dF. He, Y. Bo, J. D. Altom, E. J. Corey, J. Am. Chem. Soc. 1999, 121, 6771;
- 3eS. Lucarini, F. Bartoccini, F. Battistoni, G. Diamantini, G. Piersanti, M. Righi, G. Spadoni, Org. Lett. 2010, 12, 3844;
- 3fT. Newhouse, C. A. Lewis, K. J. Eastman, P. S. Baran, J. Am. Chem. Soc. 2010, 132, 7119;
- 3gZ.-W. Zuo, W.-Q. Xie, D.-W. Ma, J. Am. Chem. Soc. 2010, 132, 13226;
- 3hH.-X. Wu, F. Xue, X. Xiao, Y. Qin, J. Am. Chem. Soc. 2010, 132, 14052.
- 4
- 4aJ. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao, D. W. C. MacMillan, Proc. Natl. Acad. Sci. USA 2004, 101, 5482;
- 4bB. M. Trost, J. Quancard, J. Am. Chem. Soc. 2006, 128, 6314;
- 4cS. B. Jones, B. Simmons, D. W. C. MacMillan, J. Am. Chem. Soc. 2009, 131, 13606;
- 4dL. M. Repka, J. Ni, S. E. Reisman, J. Am. Chem. Soc. 2010, 132, 14418;
- 4eC.-W. Zheng, Y.-P. Lu, J.-K. Zhang, X.-K. Chen, Z. Chai, W.-Y. Ma, G. Zhao, Chem. Eur. J. 2010, 16, 5853;
- 4fR. R. Knowles, J. Carpenter, S. B. Blakey, A. Kayano, I. K. Mangion, C. J. Sinz, D. W. C. MacMillan, Chem. Sci. 2011, 2, 308; for a recent racemic example, see:
- 4gG. Cera, P. Crispino, M. Monari, M. Bandini, Chem. Commun. 2011, DOI: .
- 5
- 5aT. M. Kutchan, The Alkaloids: Chemistry and Biology, Vol. 50 (Ed.: ), Academic Press, London, 1998, pp. 257–316;
10.1016/S1099-4831(08)60045-0 Google Scholar
- 5bP. R. Blakemore, J. D. White, Chem. Commun. 2002, 1159;
- 5cG. Dondio, S. Ronzoni, D. S. Eggleston, M. Artico, P. Petrillo, G. Petrone, L. Visentin, C. Farina, V. Vecchietti, G. D. Clarke, J. Med. Chem. 1997, 40, 3192;
- 5dR. Sakai, T. Higa, C. W. Jefford, G. Bernardinelli, J. Am. Chem. Soc. 1986, 108, 6404;
- 5eJ. Kobayashi, M. Tsuda, M. Ishibashi, Pure Appl. Chem. 1999, 71, 1123;
- 5fJ. D. Winkler, J. M. Axten, J. Am. Chem. Soc. 1998, 120, 6425;
- 5gS. F. Martin, J. M. Humphrey, A. Ali, M. C. Hillier, J. Am. Chem. Soc. 1999, 121, 866.
- 6
- 6aQ. Cai, Z.-A. Zhao, S.-L. You, Angew. Chem. 2009, 121, 7564; Angew. Chem. Int. Ed. 2009, 48, 7428;
- 6bQ. Cai, C. Zheng, S.-L. You, Angew. Chem. 2010, 122, 8848; Angew. Chem. Int. Ed. 2010, 49, 8666.
- 7For a KOtBu-catalyzed anionic polycyclization of indole:
- 7aL. Turet, I. E. Markó, B. Tinant, J.-P. Declercq, R. Touillaux, Tetrahedron Lett. 2002, 43, 6591;
- 7bN. Heureux, J. Wouters, I. E. Markó, Org. Lett. 2005, 7, 5254;
- 7cN. Heureux, J. Wouters, B. Norberg, I. E. Markó, Org. Biomol. Chem. 2006, 4, 3898.
- 8A chiral primary amine salt was first applied as an iminium catalyst by Ishihara and co-workers:
- 8aK. Ishihara, K. Nakano, J. Am. Chem. Soc. 2005, 127, 10504;
- 8bA. Sakakura, K. Suzuki, K. Nakano, K. Ishihara, Org. Lett. 2006, 8, 2229;
- 8cA. Sakakura, K. Suzuki, K. Ishihara, Adv. Synth. Catal. 2006, 348, 2457;
- 8dK. Ishihara, K. Nakano, J. Am. Chem. Soc. 2007, 129, 8930;
- 8eK. Ishihara, A. Sakakura, M. Hatano, Synlett 2007, 686;
- 8fK. Ishihara, K. Nakano, M. Akakura, Org. Lett. 2008, 10, 2893; For reviews on chiral primary amine catalysis, see:
- 8gG. Bartoli, P. Melchiorre, Synlett 2008, 1759;
- 8hY.-C. Chen, Synlett 2008, 1919;
- 8iF. Peng, Z. Shao, J. Mol. Catal. A 2008, 285, 1;
- 8jL.-W. Xu, Y. Lu, Org. Biomol. Chem. 2008, 6, 2047;
- 8kL.-W. Xu, J. Luo, Y. Lu, Chem. Commun. 2009, 1807.
- 9For selected reviews on iminium catalysis, see:
- 9aG. Lelais, D. W. C. MacMillan, Aldrichimica Acta 2006, 39, 79;
- 9bA. Erkkila, I. Majander, P. M. Pihko, Chem. Rev. 2007, 107, 5416.
- 10For recent reviews of using iminium-enamine chemistry in cascade reactions, see:
- 10aD. Enders, C. Grondal, M. R. M. Hüttl, Angew. Chem. 2007, 119, 1567;
10.1002/ange.200790031 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1545;
- 10bX. Yu, W. Wang, Org. Biomol. Chem. 2008, 6, 2037; for selected examples, see:
- 10cT. Bui, C. F. Barbas III, Tetrahedron Lett. 2000, 41, 6951;
- 10dJ. W. Yang, M. T. Hechavarria Fonseca, B. List, J. Am. Chem. Soc. 2005, 127, 15036;
- 10eY. Huang, A. M. Walji, C. H. Larsen, D. W. C. MacMillan, J. Am. Chem. Soc. 2005, 127, 15051;
- 10fM. Marigo, T. Schulte, J. Franzén, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 15710;
- 10gW. Wang, H. Li, J. Wang, L. Zu, J. Am. Chem. Soc. 2006, 128, 10354;
- 10hB. Simmons, A. M. Walji, D. W. C. MacMillan, Angew. Chem. 2009, 121, 4413;
10.1002/ange.200900220 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4349;
- 10iX. Zhang, S. Zhang, W. Wang, Angew. Chem. 2010, 122, 1523; Angew. Chem. Int. Ed. 2010, 49, 1481.
- 11For selected iminium catalysis by cinchona-alkaloid-derived primary amines, see:
- 11aJ.-W. Xie, W. Chen, R. Li, M. Zeng, W. Du, L. Yue, Y.-C. Chen, Y. Wu, J. Zhu, J.-G. Deng, Angew. Chem. 2007, 119, 393; Angew. Chem. Int. Ed. 2007, 46, 389;
- 11bJ.-W. Xie, L. Yue, W. Chen, W. Du, J. Zhu, J.-G. Deng, Y.-C. Chen, Org. Lett. 2007, 9, 413;
- 11cW. Chen, W. Du, L. Yue, R. Li, Y. Wu, L.-S. Ding, Y.-C. Chen, Org. Biomol. Chem. 2007, 5, 816;
- 11dG. Bartoli, M. Bosco, A. Carlone, F. Pesciaioli, L. Sambri, P. Melchiorre, Org. Lett. 2007, 9, 1403;
- 11eW. Chen, W. Du, Y.-Z. Duan, Y. Wu, S.-Y. Yang, Y.-C. Chen, Angew. Chem. 2007, 119, 7811; Angew. Chem. Int. Ed. 2007, 46, 7667;
- 11fA. Carlone, G. Bartoli, M. Bosco, F. Pesciaioli, P. Ricci, L. Sambri, P. Melchiorre, Eur. J. Org. Chem. 2007, 5492;
- 11gP. Ricci, A. Carlone, G. Bartoli, M. Bosco, L. Sambri, P. Melchiorre, Adv. Synth. Catal. 2008, 350, 49;
- 11hP. Ricci, A. Carlone, G. Bartoli, M. Bosco, L. Sambri, P. Melchiorre, Org. Biomol. Chem. 2008, 6, 349;
- 11iR. P. Singh, K. Bartelson, Y. Wang, H. Su, X. Lu, L. Deng, J. Am. Chem. Soc. 2008, 130, 2422;
- 11jX. Lu, L. Deng, Angew. Chem. 2008, 120, 7824; Angew. Chem. Int. Ed. 2008, 47, 7710;
- 11kS. Gogoi, C.-G. Zhao, D. Ding, Org. Lett. 2009, 11, 2249;
- 11lM. W. Paixão, N. Holub, C. Vila, M. Nielsen, K. A. Jørgensen, Angew. Chem. 2009, 121, 7474;
10.1002/ange.200903790 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7338;
- 11mE. Zhang, C.-A. Fan, Y.-Q. Tu, F.-M. Zhang, Y.-L. Song, J. Am. Chem. Soc. 2009, 131, 14626;
- 11nC. Liu, Y. Lu, Org. Lett. 2010, 12, 2278;
- 11oJ. Lv, H. Wu, Y. Wang, Eur. J. Org. Chem. 2010, 2073;
- 11pN. Holub, H. Jiang, M. W. Paixão, C. Tiberi, K. A. Jørgensen, Chem. Eur. J. 2010, 16, 4337;
- 11qZ. Qiao, Z. Shafiq, L. Liu, Z.-B. Yu, Q. Zheng, D. Wang, Y.-J. Chen, Angew. Chem. 2010, 122, 7452; Angew. Chem. Int. Ed. 2010, 49, 7294;
- 11rX.-M. Li, B. Wang, J.-M. Zhang, M. Yan, Org. Lett. 2011, 13, 374;
- 11sJ.-B. Ling, W.-P. Wang, P.-F. Xu, ChemCatChem 2011, 3, 302; for selected enamine catalysis by cinchona-alkaloid-derived primary amines, see:
- 11tS. H. McCooey, S. J. Connon, Org. Lett. 2007, 9, 599;
- 11uT.-Y. Liu, H.-L. Cui, Y. Zhang, K. Jiang, W. Du, Z.-Q. He, Y.-C. Chen, Org. Lett. 2007, 9, 3671;
- 11vJ. Zhou, V. Wakchaure, P. Kraft, B. List, Angew. Chem. 2008, 120, 7677; Angew. Chem. Int. Ed. 2008, 47, 7565;
- 11wG. Bergonzini, S. Vera, P. Melchiorre, Angew. Chem. 2010, 122, 9879;
10.1002/ange.201004761 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9685;
- 11xP. Kwiatkowski, T. D. Beeson, J. C. Conrad, D. W. C. MacMillan, J. Am. Chem. Soc. 2011, 133, 1738;
- 11yA. Lee, A. Michrowska, S. Sulzer-Mosse, B. List, Angew. Chem. 2011, 123, 1745; Angew. Chem. Int. Ed. 2011, 50, 1707;
- 11zC. Liu, Q. Zhu, K.-W. Huang, Y. Lu, Org. Lett. 2011, 13, 2638; for selected iminium-enamine cascade catalysis by cinchona alkaloid derived primary amine, see:
- 11aaX. Wang, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2008, 130, 6070;
- 11abC. M. Reisinger, X. Wang, B. List, Angew. Chem. 2008, 120, 8232;
10.1002/ange.200803238 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8112;
- 11acF. Pesciaioli, F. De Vincentiis, P. Galzerano, G. Bencivenni, G. Bartoli, A. Mazzanti, P. Melchiorre, Angew. Chem. 2008, 120, 8831;
10.1002/ange.200803647 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8703;
- 11adX. Lu, Y. Liu, B. Sun, B. Cindric, L. Deng, J. Am. Chem. Soc. 2008, 130, 8134;
- 11aeJ. Lv, J. Zhang, Z. Lin, Y. Wang, Chem. Eur. J. 2009, 15, 972;
- 11afP. Galzerano, F. Pesciaioli, A. Mazzanti, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 8032;
10.1002/ange.200903803 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7892;
- 11agO. Lifchits, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2010, 132, 10227;
- 11ahX. Sun, F. Yu, T. Ye, X. Liang, J. Ye, Chem. Eur. J. 2011, 17, 430;
- 11aiB. Tan, N. R. Candeias, C. F. Barbas III, Nat. Chem. 2011, 3, 473; for selected enamine/iminium cascade catalysis by cinchona alkaloid derived primary amine, see:
- 11ajL.-Y. Wu, G. Bencivenni, M. Mancinelli, A. Mazzanti, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 7332; Angew. Chem. Int. Ed. 2009, 48, 7196;
- 11akC. Cassani, X. Tian, E. C. Escudero-Adán, P. Melchiorre, Chem. Commun. 2011, 47, 233;
- 11alG. Bencivenni, L.-Y. Wu, A. Mazzanti, B. Giannichi, F. Pesciaioli, M.-P. Song, G. Bartoli, P. Melchiorre, Angew. Chem. 2009, 121, 7336;
10.1002/ange.200903192 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7200; for selected Lewis base catalysis by cinchona-alkaloid-derived primary amines, see:
- 11amB. Tan, P. J. Chua, Y. Li, G. Zhong, Org. Lett. 2008, 10, 2437;
- 11anB. Tan, Z. Shi, P. J. Chua, G. Zhong, Org. Lett. 2008, 10, 3425;
- 11aoB. Tan, P. J. Chua, X. Zeng, M. Lu, G. Zhong, Org. Lett. 2008, 10, 3489.
- 12The absolute configuration was determined by the X-ray diffraction analysis of enantiopure 3 b. CCDC 808361 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13Since only two diastereoisomers were isolated, the origin of the diastereoselectivity was studied by computational method. The DFT calculations suggested that the Mannich reaction is stereospecific because of the rigid polycylic skeleton. The cis-fused [4.3.0] bicyclic structure is the only accessible product of the cyclization process. See the Supporting Information for details.
- 14The relative configurations of 3 k and 3 k′ were determined by the X-ray diffraction. CCDC 828201 and 828107 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15
- 15aJ. Fridrichsons, A. Mcl. Mathieson, M. F. Mackay, Tetrahedron 1970, 26, 1869;
- 15bT. Kametani, T. Kohno, R. Charubala, K. Fukumoto, Tetrahedron 1972, 28, 3227.
- 16J. Sapi, S. Dridi, J. Laronze, F. Sigaut, D. Patigny, J.-Y. Laronze, J. Lévy, Tetrahedron 1996, 52, 8209.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.