A Fluorescent Reporter of the Phosphorylation Status of the Substrate Protein STAT3†
Vanessa K. Lacey
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Search for more papers by this authorAngela R. Parrish
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Search for more papers by this authorShuliang Han
College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorDr. Zhouxin Shen
Section of Cell and Development Biology, University of California at San Diego, La Jolla, CA 92037 (USA)
Search for more papers by this authorProf. Steven P. Briggs
Section of Cell and Development Biology, University of California at San Diego, La Jolla, CA 92037 (USA)
Search for more papers by this authorProf. Yuguo Ma
College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorCorresponding Author
Prof. Lei Wang
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/Search for more papers by this authorVanessa K. Lacey
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Search for more papers by this authorAngela R. Parrish
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Search for more papers by this authorShuliang Han
College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorDr. Zhouxin Shen
Section of Cell and Development Biology, University of California at San Diego, La Jolla, CA 92037 (USA)
Search for more papers by this authorProf. Steven P. Briggs
Section of Cell and Development Biology, University of California at San Diego, La Jolla, CA 92037 (USA)
Search for more papers by this authorProf. Yuguo Ma
College of Chemistry, Peking University, Beijing 100871 (China)
Search for more papers by this authorCorresponding Author
Prof. Lei Wang
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/
Jack H. Skirball Center for Chemical Biology & Proteomics, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92037 (USA) http://wang.salk.edu/Search for more papers by this authorWe thank Dr. Tony Hunter for helpful discussions. L.W. thanks the support from the Salk Innovation grant, March of Dimes Foundation (5-FY08-110), CIRM (RN1-00577-1), NCI (P30CA014195) and NIH (1DP2OD004744).
Graphical Abstract
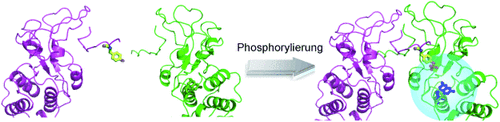
Ein reversibler Biosensor gibt Auskunft über den Phosphorylierungszustand des Volllängen-Substratproteins STAT3, indem er genetisch 7-Hydroxycumarin in dessen Phosphotyrosin-Bindetasche einbaut (siehe Bild). Eine starke Zunahme der Fluoreszenz mit charakteristischen Emissions- und Anregungseigenschaften tritt bei Phosphorylierung von STAT3 in vitro und endogen in Zellkernextrakten auf.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201102923_sm_miscellaneous_information.pdf855.8 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Z. A. Knight, H. Lin, K. M. Shokat, Nat. Rev. Cancer 2010, 10, 130–137.
- 2M. D. Shults, B. Imperiali, J. Am. Chem. Soc. 2003, 125, 14248–14249; M. D. Shults, K. A. Janes, D. A. Lauffenburger, B. Imperiali, Nat. Methods 2005, 2, 277–283; R. H. Yeh, X. Yan, M. Cammer, A. R. Bresnick, D. S. Lawrence, J. Biol. Chem. 2002, 277, 11527–11532; V. Sharma, R. S. Agnes, D. S. Lawrence, J. Am. Chem. Soc. 2007, 129, 2742–2743; D. M. Rothman, M. D. Shults, B. Imperiali, Trends Cell Biol. 2005, 15, 502–510; V. Sharma, Q. Wang, D. S. Lawrence, Biochim. Biophys. Acta Proteins Proteomics 2008, 1784, 94–99.
- 3J. Zhang, Y. Ma, S. S. Taylor, R. Y. Tsien, Proc. Natl. Acad. Sci. USA 2001, 98, 14997–15002; J. D. Violin, J. Zhang, R. Y. Tsien, A. C. Newton, J. Cell Biol. 2003, 161, 899–909; A. Y. Ting, K. H. Kain, R. L. Klemke, R. Y. Tsien, Proc. Natl. Acad. Sci. USA 2001, 98, 15003–15008; Y. Wang, E. L. Botvinick, Y. Zhao, M. W. Berns, S. Usami, R. Y. Tsien, S. Chien, Nature 2005, 434, 1040–1045; K. M. Humphries, J. K. Pennypacker, S. S. Taylor, J. Biol. Chem. 2007, 282, 22072–22079; M. Ouyang, H. Huang, N. C. Shaner, A. G. Remacle, S. A. Shiryaev, A. Y. Strongin, R. Y. Tsien, Y. Wang, Cancer Res. 2010, 70, 2204–2212; J. Lippincott-Schwartz, E. Snapp, A. Kenworthy, Nat. Rev. Mol. Cell Biol. 2001, 2, 444–456; J. Zhang, R. E. Campbell, A. Y. Ting, R. Y. Tsien, Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918; A. Miyawaki, Dev. Cell 2003, 4, 295–305; J. Zhang, M. D. Allen, Mol. Biosyst. 2007, 3, 759–765.
- 4A. Remenyi, M. C. Good, W. A. Lim, Curr. Opin. Struct. Biol. 2006, 16, 676–685.
- 5G. Manning, D. B. Whyte, R. Martinez, T. Hunter, S. Sudarsanam, Science 2002, 298, 1912–1934.
- 6H. Yu, D. Pardoll, R. Jove, Nat. Rev. Cancer 2009, 9, 798–809.
- 7D. W. Fink, W. R. Koehler, Anal. Chem. 1970, 42, 990–993.
- 8S. Becker, B. Groner, C. W. Muller, Nature 1998, 394, 145–151.
- 9J. Wang, J. Xie, P. G. Schultz, J. Am. Chem. Soc. 2006, 128, 8738–8739.
- 10A. K. Kretzschmar, M. C. Dinger, C. Henze, K. Brocke-Heidrich, F. Horn, Biochem. J. 2004, 377, 289–297.
- 11B. J. Mayer, P. K. Jackson, R. A. Van Etten, D. Baltimore, Mol. Cell. Biol. 1992, 12, 609–618.
- 12Z. Ren, X. Mao, C. Mertens, R. Krishnaraj, J. Qin, P. K. Mandal, M. J. Romanowski, J. S. McMurray, X. Chen, Biochem. Biophys. Res. Commun. 2008, 374, 1–5.
- 13T. Moriya, Bull. Chem. Soc. Jpn. 1983, 56, 6–14.
- 14P. E. Zinsli, J. Photochem. 1974, 3, 55–69.
- 15C. Lutticken et al., Science 1994, 263, 89–92.
- 16O. K. Park, L. K. Schaefer, W. Wang, T. S. Schaefer, J. Biol. Chem. 2000, 275, 32244–32249.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.