The 3D Solution Structure of Thurincin H, a Bacteriocin with Four Sulfur to α-Carbon Crosslinks†
We thank Dr. Randy Whittal, Jing Zheng, and Bela Reiz for performing the mass spectrometry analysis. We thank Mark Miskolzie for advice regarding NMR experiments and data analysis. We thank Dr. Leah Martin-Visscher and Dr. Pascal Mercier for their assistance with CYANA. This research was supported by the Alberta Scholarship Programs (to C.S.S.), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Research Chair in Bioorganic and Medicinal Chemistry, the Alberta Heritage Foundation for Medical Research (AHFMR), and the United States Department of Agriculture—National Integrated Food Safety Initiative (USDA-NIFSI) Grant no. 2008-51110-0688.
Graphical Abstract
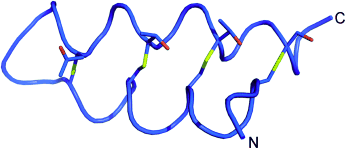
Einblick ins Peptid: Für das antimikrobielle Peptid Thurincin H wurde eine Modifizierung nach der Translation vermutet. Eine MS/MS-Sequenzierung identifizierte nun die modifizierten Reste, und NMR-spektroskopische Studien in Lösung ergaben eine 3D-Struktur für Thurincin H, in der vier S-Cα-Thioether-Brücken auftreten (gelb im Bild). Diese Struktur könnte auch für einige andere Bacteriocine mit identischer Masse bedeutsam sein.