Generation of Melamine Polymer Condensates upon Hypergolic Ignition of Dicyanamide Ionic Liquids†
Dr. Konstantin Chingin
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Search for more papers by this authorDr. Richard H. Perry
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Search for more papers by this authorDr. Steven D. Chambreau
Air Force Research Laboratory, AFRL/RZSP, Edwards Air Force Base, 10 East Saturn Boulevard, CA 93524 (USA)
Search for more papers by this authorDr. Ghanshyam L. Vaghjiani
Air Force Research Laboratory, AFRL/RZSP, Edwards Air Force Base, 10 East Saturn Boulevard, CA 93524 (USA)
Search for more papers by this authorCorresponding Author
Prof. Richard N. Zare
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)Search for more papers by this authorDr. Konstantin Chingin
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Search for more papers by this authorDr. Richard H. Perry
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Search for more papers by this authorDr. Steven D. Chambreau
Air Force Research Laboratory, AFRL/RZSP, Edwards Air Force Base, 10 East Saturn Boulevard, CA 93524 (USA)
Search for more papers by this authorDr. Ghanshyam L. Vaghjiani
Air Force Research Laboratory, AFRL/RZSP, Edwards Air Force Base, 10 East Saturn Boulevard, CA 93524 (USA)
Search for more papers by this authorCorresponding Author
Prof. Richard N. Zare
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)
Department of Chemistry, Stanford University, 333 Campus Drive, Stanford, CA 94305-5080 (USA)Search for more papers by this authorThis work has been supported by the Air Force Office of Scientific Research (AFOSR: FA 9550-10-1-0235) and the Center for Molecular Analysis and Design at Stanford University (CMAD: 1123893-1-AABGE). K.C. acknowledges financial help from the Swiss National Science Foundation (PBEZP2-133126). We thank Prof. Wolfgang Schnick (LMU Munich) and Dr. Joseph Mabry (AFRL, Edwards Air Force Base) for helpful discussions on nitride chemistry. We also thank Dr. Pavel Aronov and Dr. Allis Chien (Stanford University Mass Spectrometry) for providing instrumentation and their expertise in the field of mass spectrometry. Dr. Jun Ge (Stanford University) is acknowledged for his help with SEM measurements.
Graphical Abstract
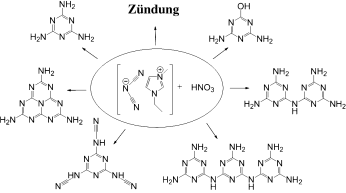
Die Reaktion von ionischen Flüssigkeiten mit Dicyanamid-Ionen mit Salpetersäure hat eine hypergole Zündung und die Bildung eines stabilen Niederschlags zur Folge. Dieser besteht aus cyclischen Triazinen, darunter Melamin und seine Polymere wie Melam und Melem (siehe Schema). Diese Studie führt einen neuen Syntheseansatz für cyclische Azine ein, der hohe Temperaturen und Drücke umgeht.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201101247_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Tanaka, W. Daimon, I. Kimura, J. Propul. Power 1985, 1, 314.
- 2
- 2aT. M. Klapotke, G. Holl, Green Chem. 2001, 3, G 75;
- 2bM. B. Talawar, R. Sivabalan, T. Mukundan, H. Muthurajan, A. K. Sikder, B. R. Gandhe, A. S. Rao, J. Hazard. Mater. 2009, 161, 589.
- 3
- 3aJ. H. Davis, Chem. Lett. 2004, 33, 1072;
- 3bP. Wasserscheid, W. Keim, Angew. Chem. 2000, 112, 3926;
10.1002/1521-3757(20001103)112:21<3926::AID-ANGE3926>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3772;10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 3cJ. Dupont, R. F. de Souza, P. A. Z. Suarez, Chem. Rev. 2002, 102, 3667.
- 4
- 4aM. Smiglak, A. Metlen, R. D. Rogers, Acc. Chem. Res. 2007, 40, 1182;
- 4bM. Freemantle, Chem. Eng. News 2007, 85, 23;
- 4cR. P. Singh, R. D. Verma, D. T. Meshri, J. M. Shreeve, Angew. Chem. 2006, 118, 3664; Angew. Chem. Int. Ed. 2006, 45, 3584.
- 5S. Schneider, T. Hawkins, M. Rosander, G. Vaghjiani, S. Chambreau, G. Drake, Energy Fuel 2008, 22, 2871.
- 6S. D. Chambreau, S. Schneider, M. Rosander, T. Hawkins, C. J. Gallegos, M. F. Pastewait, G. L. Vaghjiani, J. Phys. Chem. A 2008, 112, 7816.
- 7
- 7aH. X. Gao, Y. H. Joo, B. Twamley, Z. Q. Zhou, J. M. Shreeve, Angew. Chem. 2009, 121, 2830; Angew. Chem. Int. Ed. 2009, 48, 2792;
- 7bL. He, G. H. Tao, D. A. Parrish, J. M. Shreeve, Chem. Eur. J. 2010, 16, 5736;
- 7cY. H. Joo, H. X. Gao, Y. Q. Zhang, J. M. Shreeve, Inorg. Chem. 2010, 49, 3282;
- 7dY. Q. Zhang, H. X. Gao, Y. Guo, Y. H. Joo, J. M. Shreeve, Chem. Eur. J. 2010, 16, 3114;
- 7eY. Q. Zhang, J. M. Shreeve, Angew. Chem. 2011, 123, 965; Angew. Chem. Int. Ed. 2011, 50, 935.
- 8
- 8aY. Yoshida, K. Muroi, A. Otsuka, G. Saito, M. Takahashi, T. Yoko, Inorg. Chem. 2004, 43, 1458;
- 8bD. R. MacFarlane, J. Golding, S. Forsyth, M. Forsyth, G. B. Deacon, Chem. Commun. 2001, 1430.
- 9
- 9aS. S. Ju, C. C. Han, C. J. Wu, A. M. Mebel, Y. T. Chen, J. Phys. Chem. B 1999, 103, 582;
- 9bL. Zhu, G. Gamez, H. W. Chen, K. Chingin, R. Zenobi, Chem. Commun. 2009, 559.
- 10B. Jurgens, H. A. Hoppe, E. Irran, W. Schnick, Inorg. Chem. 2002, 41, 4849.
- 11B. V. Lotsch, W. Schnick, New J. Chem. 2004, 28, 1129.
- 12B. V. Lotsch, W. Schnick, Chem. Eur. J. 2007, 13, 4956.
- 13B. Jurgens, E. Irran, J. Senker, P. Kroll, H. Muller, W. Schnick, J. Am. Chem. Soc. 2003, 125, 10288.
- 14A. Sattler, S. Pagano, M. Zeuner, A. Zurawski, D. Gunzelmann, J. Senker, K. Muller-Buschbaum, W. Schnick, Chem. Eur. J. 2009, 15, 13161.
- 15M. Takimoto, Kogyo Kagaku Zasshi 1961, 64, 1452.
- 16C. E. Stoner, T. B. Brill, Combust. Flame 1991, 83, 302.
- 17G. K. Williams, S. F. Palopoli, T. B. Brill, Combust. Flame 1994, 98, 197.
- 18
- 18aJ. P. Paraknowitsch, A. Thomas, M. Antonietti, J. Mater. Chem. 2010, 20, 6746;
- 18bJ. P. Paraknowitsch, J. Zhang, D. S. Su, A. Thomas, M. Antonietti, Adv. Mater. 2010, 22, 87;
- 18cT. J. Wooster, K. M. Johanson, K. J. Fraser, D. R. MacFarlane, J. L. Scott, Green Chem. 2006, 8, 691.
- 19
- 19aC. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. Aki, J. F. Brennecke, J. Chem. Eng. Data 2004, 49, 954;
- 19bM. Kosmulski, J. Gustafsson, J. B. Rosenholm, Thermochim. Acta 2004, 412, 47;
- 19cK. J. Baranyai, G. B. Deacon, D. R. MacFarlane, J. M. Pringle, J. L. Scott, Aust. J. Chem. 2004, 57, 145.
- 20J. Geith, G. Holl, T. M. Klapotke, J. J. Weigand, Combust. Flame 2004, 139, 358.
- 21
- 21aG. Fischer, J. Geith, T. M. Klapotke, B. Krumm, Z. Naturforsch. B 2002, 57, 19;
- 21bD. J. Belson, A. N. Strachan, Chem. Soc. Rev. 1982, 11, 41.
- 22
- 22aE. Irran, B. Jurgens, W. Schnick, Chem. Eur. J. 2001, 7, 5372;
10.1002/1521-3765(20011217)7:24<5372::AID-CHEM5372>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 22bW. Madelung, E. Kern, Justus Liebigs Ann. Chemie 1922, 427, 26;
- 22cA. P. Purdy, E. Houser, C. F. George, Polyhedron 1997, 16, 3671.
- 23
- 23aB. V. Lotsch, W. Schnick, Chem. Mater. 2005, 17, 3976;
- 23bB. V. Lotsch, W. Schnick, Chem. Mater. 2006, 18, 1891;
- 23cZ. H. Zhang, K. Leinenweber, M. Bauer, L. A. J. Garvie, P. F. McMillan, G. H. Wolf, J. Am. Chem. Soc. 2001, 123, 7788;
- 23dD. R. Miller, D. C. Swenson, E. G. Gillan, J. Am. Chem. Soc. 2004, 126, 5372;
- 23eJ. R. Holst, E. G. Gillan, J. Am. Chem. Soc. 2008, 130, 7373.
- 24
- 24aR. H. Perry, R. G. Cooks, R. J. Noll, Mass Spectrom. Rev. 2008, 27, 661;
- 24bQ. Z. Hu, R. J. Noll, H. Y. Li, A. Makarov, M. Hardman, R. G. Cooks, J. Mass Spectrom. 2005, 40, 430;
- 24cA. Makarov, E. Denisov, A. Kholomeev, W. Baischun, O. Lange, K. Strupat, S. Horning, Anal. Chem. 2006, 78, 2113.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.