Enantioselective Total Syntheses of Three Cladiellins (Eunicellins): A General Approach to the Entire Family of Natural Products†
Corresponding Author
Prof. Dr. J. Stephen Clark
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867Search for more papers by this authorRaphaëlle Berger
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
Search for more papers by this authorDr. Stewart T. Hayes
School of Chemistry, University of Nottingham, University Park, Nottingham NG7 2RD (United Kingdom)
Search for more papers by this authorDr. Lynne H. Thomas
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
Search for more papers by this authorDr. Angus J. Morrison
MSD, Newhouse, Lanarkshire, ML1 5SH (United Kingdom)
Search for more papers by this authorDr. Luca Gobbi
Discovery Chemistry (PRCB), F. Hoffmann-La Roche Ltd, Pharmaceuticals Division, 4070 Basel (Switzerland)
Search for more papers by this authorCorresponding Author
Prof. Dr. J. Stephen Clark
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867Search for more papers by this authorRaphaëlle Berger
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
Search for more papers by this authorDr. Stewart T. Hayes
School of Chemistry, University of Nottingham, University Park, Nottingham NG7 2RD (United Kingdom)
Search for more papers by this authorDr. Lynne H. Thomas
WestCHEM, School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow G12 8QQ (United Kingdom), Fax: (+44) 141-330-6867
Search for more papers by this authorDr. Angus J. Morrison
MSD, Newhouse, Lanarkshire, ML1 5SH (United Kingdom)
Search for more papers by this authorDr. Luca Gobbi
Discovery Chemistry (PRCB), F. Hoffmann-La Roche Ltd, Pharmaceuticals Division, 4070 Basel (Switzerland)
Search for more papers by this authorWe thank the EPSRC, M.S.D. and F. Hoffmann-La Roche Ltd for financial support.
Graphical Abstract
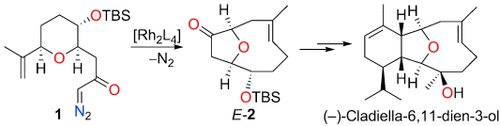
Durch stereoselektive Umlagerung eines freien oder metallgebundenen Oxonium-Ylids, das durch rhodiumkatalysierte intramolekulare Cyclisierung des Diazoketons 1 erzeugt wurde, entstand der E-konfigurierte, O-verbrückte, bicyclische Ether (E)-2, der effizient in (−)-Cladiella-6,11-dien-3-ol überführt wurde. TBS=tert-Butyldimethylsilyl.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201005508_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews concerning the isolation of the cladiellins and related marine diterpene natural products, see:
- 1aP. Bernardelli, L. A. Paquette, Heterocycles 1998, 49, 531–556;
- 1bP.-S. Sung, M.-C. Chen, Heterocycles 2002, 57, 1705–1715.
- 2
- 2aD. W. C. MacMillan, L. E. Overman, J. Am. Chem. Soc. 1995, 117, 10391–10392;
- 2bL. E. Overman, L. D. Pennington, Org. Lett. 2000, 2, 2683–2686;
- 2cF. Gallou, D. W. C. MacMillan, L. E. Overman, L. A. Paquette, L. D. Pennington, J. Yang, Org. Lett. 2001, 3, 135–137;
- 2dP. Bernardelli, O. M. Moradei, D. Friedrich, J. Yang, F. Gallou, B. P. Dyck, R. W. Doskotch, T. Lange, L. A. Paquette, J. Am. Chem. Soc. 2001, 123, 9021–9032;
- 2eD. W. C. MacMillan, L. E. Overman, L. D. Pennington, J. Am. Chem. Soc. 2001, 123, 9033–9044;
- 2fO. Corminboeuf, L. E. Overman, L. D. Pennington, J. Am. Chem. Soc. 2003, 125, 6650–6652;
- 2gG. A. Molander, D. J. St. Jean, Jr., J. Haas, J. Am. Chem. Soc. 2004, 126, 1642–1643;
- 2hM. T. Crimmins, B. H. Brown, J. Am. Chem. Soc. 2004, 126, 10264–10266;
- 2iM. T. Crimmins, J. M. Ellis, J. Am. Chem. Soc. 2005, 127, 17200–17201;
- 2jM. T. Crimmins, B. H. Brown, H. R. Plake, J. Am. Chem. Soc. 2006, 128, 1371–1378;
- 2kH. Kim, H. Lee, J. Kim, S. Kim, D. Kim, J. Am. Chem. Soc. 2006, 128, 15851–15855;
- 2lJ. Becker, K. Bergander, R. Froehlich, D. Hoppe, Angew. Chem. 2008, 120, 1678–1681;
10.1002/ange.200704678 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1654–1657;
- 2mM. T. Crimmins, J. M. Ellis, J. Org. Chem. 2008, 73, 1649–1660;
- 2nO. Corminboeuf, L. E. Overman, L. D. Pennington, J. Org. Chem. 2009, 74, 5458–5470;
- 2oM. J. Campbell, J. S. Johnson, J. Am. Chem. Soc. 2009, 131, 10370–10371.
- 3For a recent review of strategies used to synthesize the cladiellins and related marine diterpene natural products, see: J. M. Ellis, M. T. Crimmins, Chem. Rev. 2008, 108, 5278–5298.
- 4J. S. Clark, S. T. Hayes, C. Wilson, L. Gobbi, Angew. Chem. 2007, 119, 441–444; Angew. Chem. Int. Ed. 2007, 46, 437–440.
- 5J. E. Hochlowski, D. J. Faulkner, Tetrahedron Lett. 1980, 21, 4055–4056.
- 6Y. Seo, J.-R. Rho, K. W. Cho, J. Shin, J. Nat. Prod. 1997, 60, 171–174.
- 7Y. Uchio, M. Nakatani, T. Hase, M. Kodama, S. Usui, Y. Fukazawa, Tetrahedron Lett. 1989, 30, 3331–3332.
- 8
- 8aA. Padwa, S. F. Hornbuckle, Chem. Rev. 1991, 91, 263–309;
- 8bT. Ye, A. M. McKervey, Chem. Rev. 1994, 94, 1091–1160;
- 8cJ. S. Clark in Nitrogen, Oxygen and Sulfur Ylide Chemistry, A Practical Approach in Chemistry (Ed: ), Oxford University Press, Oxford, 2002, chap. 1, pp. 1–113.
- 9
- 9aJ. S. Clark, Tetrahedron Lett. 1992, 33, 6193–6196;
- 9bJ. S. Clark, S. A. Krowiak, L. J. Street, Tetrahedron Lett. 1993, 34, 4385–4388;
- 9cF. G. West, B. N. Naidu, R. W. Tester, J. Org. Chem. 1994, 59, 6892–6894;
- 9dJ. S. Clark, G. Whitlock, S. Jiang, N. Onyia, Chem. Commun. 2003, 2578–2579;
- 9eF. P. Marmsäter, J. A. Vanecko, F. G. West, Org. Lett. 2004, 6, 1657–1660;
- 9fJ. S. Clark, S. B. Walls, C. Wilson, S. P. East, M. J. Drysdale, Eur. J. Org. Chem. 2006, 323–327;
- 9gJ. S. Clark, C. Guérot, C. Wilson, A. J. Blake, Chem. Commun. 2007, 4134–4136.
- 10D. R. Williams, K. G. Meyer, K. Shamim, S. Patnaik, Can. J. Chem. 2004, 82, 120–130.
- 11
- 11aE. J. Corey, R. K. Bakshi, S. Shibata, J. Am. Chem. Soc. 1987, 109, 5551–5553;
- 11bE. J. Corey, C. J. Helal, Angew. Chem. 1998, 110, 2092–2118;
10.1002/(SICI)1521-3757(19980803)110:15<2092::AID-ANGE2092>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1986–2012;10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 11cS. Wallbaum, J. Martens, Tetrahedron: Asymmetry 1992, 3, 1475–1504.
- 12
- 12aG. Matsuo, H. Kadohama, T. Nakata, Chem. Lett. 2002, 148–149;
- 12bJ. S. Clark, S. T. Hayes, A. J. Blake, L. Gobbi, Tetrahedron Lett. 2007, 48, 2501–2503.
- 13H. B. Kwon, B. H. McKee, J. K. Stille, J. Org. Chem. 1990, 55, 3114–3118.
- 14Complete facial selectivity was observed with respect to the diene during the thermal Diels–Alder reaction (9→10, Scheme 3). Decomposition of diene 9 was observed when a Lewis acid catalyst was added to the Diels–Alder reaction.
- 15A. G. M. Barrett, C. R. A. Godfrey, D. M. Hollinshead, P. A. Procopiou, D. H. R. Barton, R. B. Boar, L. Joukhadar, J. F. McGhie, S. C. Misra, J. Chem. Soc. Perkin Trans. 1 1981, 1501–1509.
- 16G. Giuffredi, C. Bobbio, V. Gouverneur, J. Org. Chem. 2006, 71, 5361–5364.
- 17Crystal data for cladiell-11-ene-3,6,7-triol: C20H34O4, Mr=338.47, crystal dimensions 0.30×0.1×0.05 mm3, orthorhombic, space group P212121, a=6.9388(6), b=8.1850(10), c=33.548(3) Å, α=β=γ=90°, V=1905.3(3) Å3, Z=4, ρcalcd=1.18 Mg m−3, μ(MoKα)=0.08 mm−1, T=100(2) K.; 13 485 reflections collected of which 4356 were independent, 2θmax=55°. Structure solved by direct methods (SHELXS-97) and refined by full-matrix least squares against F2 (SHELXL-97), R1=0.074, wR2=0.096, 225 parameters. CCDC 787739 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.