Rhodium-Catalyzed Asymmetric Synthesis of Spirocarbocycles: Arylboron Reagents as Surrogates of 1,2-Dimetalloarenes†
Ryo Shintani Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorShingo Isobe
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorMomotaro Takeda
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorTamio Hayashi Prof. Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorRyo Shintani Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorShingo Isobe
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorMomotaro Takeda
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorTamio Hayashi Prof. Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502 (Japan), Fax: (+81) 75-753-3988
Search for more papers by this authorSupport has been provided in part by a Grant-in-Aid for Scientific Research (S; 19105002) from the Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Graphical Abstract
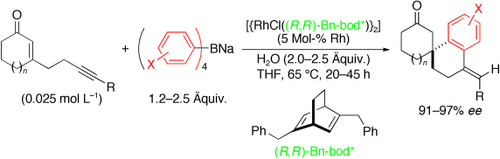
Von Eninen zu Spirocyclen: Eine Rhodium-Dien-katalysierte Addition von Natriumtetraarylboraten an 2-Cycloalken-1-one mit einer anhängenden Alkineinheit führt zu Spirocarbocyclen. Dabei werden zwei neue C-C-Bindungen durch das Tetraarylborat gebildet, und der chirale Dienligand sorgt für den hoch enantioselektiven Aufbau quartärer Kohlenstoffspirozentren.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_201000937_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on quaternary stereocenters, see:
- 1aB. M. Trost, C. Jiang, Synthesis 2006, 369;
- 1bJ. Christoffers, A. Baro, Adv. Synth. Catal. 2005, 347, 1473;
- 1cC. J. Douglas, L. E. Overman, Proc. Natl. Acad. Sci. USA 2004, 101, 5363;
- 1dI. Denissova, L. Barriault, Tetrahedron 2003, 59, 10105.
- 2
- 2aG. H. Posner, T. G. Hamill, J. Org. Chem. 1988, 53, 6031;
- 2bJ. R. Hwu, J. M. Wetzel, J. Org. Chem. 1992, 57, 922;
- 2cY. Kita, H. Fujioka, Pure Appl. Chem. 2007, 79, 701.
- 3
- 3aY. Endo, S. Takehana, M. Ohno, P. E. Driedger, S. Stabel, M. Y. Mizutani, N. Tomioka, A. Itai, K. Shudo, J. Med. Chem. 1998, 41, 1476;
- 3bZ. Xiao, L. S. Kappen, I. H. Goldberg, Bioorg. Med. Chem. Lett. 2006, 16, 2895.
- 4For reviews, see:
- 4aK. Ding, Z. Han, Z. Wang, Chem. Asian J. 2009, 4, 32;
- 4bJ.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581; For leading references, see:
- 4cY.-Z. Zhang, S.-F. Zhu, L.-X. Wang, Q.-L. Zhou, Angew. Chem. 2008, 120, 8642; Angew. Chem. Int. Ed. 2008, 47, 8469;
- 4dY. Yang, S.-F. Zhu, C.-Y. Zhou, Q.-L. Zhou, J. Am. Chem. Soc. 2008, 130, 14052;
- 4eW. Shu, Q. Yu, S. Ma, Adv. Synth. Catal. 2009, 351, 2807;
- 4fT. Tsujihara, K. Takenaka, K. Onitsuka, M. Hatanaka, H. Sasai, J. Am. Chem. Soc. 2009, 131, 3452;
- 4gZ. Han, Z. Wang, X. Zhang, K. Ding, Angew. Chem. 2009, 121, 5449; Angew. Chem. Int. Ed. 2009, 48, 5345.
- 5For examples of intermolecular reactions, see:
- 5aK. Tsuchikama, Y. Kuwata, T. Shibata, J. Am. Chem. Soc. 2006, 128, 13686;
- 5bB. M. Trost, N. Cramer, S. M. Silverman, J. Am. Chem. Soc. 2007, 129, 12396;
- 5cM. Ichinose, H. Suematsu, T. Katsuki, Angew. Chem. 2009, 121, 3167;
10.1002/ange.200805527 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3121; For examples of intramolecular reactions, see:
- 5dY. Yamaura, M. Hyakutake, M. Mori, J. Am. Chem. Soc. 1997, 119, 7615;
- 5eA. Ashimori, B. Bachand, L. E. Overman, D. J. Poon, J. Am. Chem. Soc. 1998, 120, 6477;
- 5fM. Hatano, K. Mikami, J. Am. Chem. Soc. 2003, 125, 4704;
- 5gA. Wada, K. Noguchi, M. Hirano, K. Tanaka, Org. Lett. 2007, 9, 1295; See also:
- 5hX. Teng, D. R. Cefalo, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 10779;
- 5iE. Zhang, C.-A. Fan, Y.-Q. Tu, F.-M. Zhang, Y.-L. Song, J. Am. Chem. Soc. 2009, 131, 14626.
- 6
- 6aH. J. S. Winkler, G. Wittig, J. Org. Chem. 1963, 28, 1733;
- 6bM. A. G. M. Tinga, G. Schat, O. S. Akkerman, F. Bickelhaupt, E. Hora, H. Kooijman, W. J. J. Smeets, A. L. Spek, J. Am. Chem. Soc. 1993, 115, 2808;
- 6cM. Amano, A. Saiga, R. Ikegami, T. Ogata, K. Takagi, Tetrahedron Lett. 1998, 39, 8667;
- 6dA. B. Evnin, D. Seyferth, J. Am. Chem. Soc. 1967, 89, 952;
- 6eR. Altmann, K. Jurkschat, M. Schürmann, Organometallics 1998, 17, 5858;
- 6fA. B. Chopa, M. T. Lockhart, G. F. Silbestri, Organometallics 2002, 21, 5874;
- 6gH. Yoshida, K. Tanino, J. Ohshita, A. Kunai, Angew. Chem. 2004, 116, 5162; Angew. Chem. Int. Ed. 2004, 43, 5052;
- 6hK. Nozaki, M. Yoshida, H. Takaya, Bull. Chem. Soc. Jpn. 1996, 69, 2043;
- 6iM. Shimizu, I. Nagao, Y. Tomioka, T. Hiyama, Angew. Chem. 2008, 120, 8216; Angew. Chem. Int. Ed. 2008, 47, 8096;
- 6jH. Noguchi, T. Shioda, C.-M. Chou, M. Suginome, Org. Lett. 2008, 10, 377;
- 6kH. Ihara, M. Suginome, J. Am. Chem. Soc. 2009, 131, 7502;
- 6lH. Yoshida, J. Ikadai, M. Shudo, J. Ohshita, A. Kunai, J. Am. Chem. Soc. 2003, 125, 6638;
- 6mA. Kawachi, A. Tani, K. Machida, Y. Yamamoto, Organometallics 2007, 26, 4697.
- 7
- 7aT. Hayashi, K. Inoue, N. Taniguchi, M. Ogasawara, J. Am. Chem. Soc. 2001, 123, 9918;
- 7bT. Miura, T. Sasaki, H. Nakazawa, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1390;
- 7cR. Shintani, K. Takatsu, T. Hayashi, Angew. Chem. 2007, 119, 3809;
10.1002/ange.200700226 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3735; See also:
- 7dK. Oguma, M. Miura, T. Satoh, M. Nomura, J. Am. Chem. Soc. 2000, 122, 10464;
- 7eR. Shintani, K. Okamoto, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 2872;
- 7fH. Yamabe, A. Mizuno, H. Kusama, N. Iwasawa, J. Am. Chem. Soc. 2005, 127, 3248;
- 7gT. Matsuda, M. Shigeno, M. Murakami, J. Am. Chem. Soc. 2007, 129, 12086;
- 7hM. Shigeno, T. Yamamoto, M. Murakami, Chem. Eur. J. 2009, 15, 12929;
- 7iT. Seiser, O. A. Roth, N. Cramer, Angew. Chem. 2009, 121, 6438;
10.1002/ange.200903189 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6320.
- 8For examples of palladium-catalyzed transformations involving a 1,4-palladium migration, see:
- 8aM. A. Campo, R. C. Larock, J. Am. Chem. Soc. 2002, 124, 14326;
- 8bM. A. Campo, Q. Huang, T. Yao, Q. Tian, R. C. Larock, J. Am. Chem. Soc. 2003, 125, 11506;
- 8cQ. Huang, M. A. Campo, T. Yao, Q. Tian, R. C. Larock, J. Org. Chem. 2004, 69, 8251;
- 8dJ. Zhao, R. C. Larock, Org. Lett. 2005, 7, 701.
- 9For reviews on rhodium-catalyzed arylative cyclization reactions of alkyne-tethered electrophiles, see:
- 9aT. Miura, M. Murakami, Chem. Commun. 2007, 217;
- 9bS. W. Youn, Eur. J. Org. Chem. 2009, 2597; For leading references, see:
- 9cT. Miura, M. Shimada, M. Murakami, J. Am. Chem. Soc. 2005, 127, 1094;
- 9dT. Miura, T. Sasaki, T. Harumashi, M. Murakami, J. Am. Chem. Soc. 2006, 128, 2516;
- 9eT. Miura, K. Ueda, Y. Takahashi, M. Murakami, Chem. Commun. 2008, 5366;
- 9fR. Shintani, K. Okamoto, Y. Otomaru, K. Ueyama, T. Hayashi, J. Am. Chem. Soc. 2005, 127, 54;
- 9gR. Shintani, A. Tsurusaki, K. Okamoto, T. Hayashi, Angew. Chem. 2005, 117, 3977;
10.1002/ange.200500843 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3909;
- 9hY. Chen, C. Lee, J. Am. Chem. Soc. 2006, 128, 15598; See also:
- 9iD. F. Cauble, J. D. Gipson, M. J. Krische, J. Am. Chem. Soc. 2003, 125, 1110;
- 9jM. Lautens, T. Marquardt, J. Org. Chem. 2004, 69, 4607;
- 9kM. Miyamoto, Y. Harada, M. Tobisu, N. Chatani, Org. Lett. 2008, 10, 2975.
- 10
- 10aM. Ueda, N. Miyaura, J. Organomet. Chem. 2000, 595, 31;
- 10bK. Ueura, S. Miyamura, T. Satoh, M. Miura, J. Organomet. Chem. 2006, 691, 2821.
- 11The use of alkene-tethered enone 3-(4-phenyl-3-buten-1-yl)-2-cyclohexen-1-one as a substrate results in a messy reaction with approximately 40 % recovery of the substrate.
- 12kH/kD=1.04 was observed when the reaction of 1 a was conducted with an equimolar mixture of 2 a and [D20]-2 a.
- 13For reviews on rhodium-catalyzed 1,4-addition reactions, see:
- 13aJ. Christoffers, G. Koripelly, A. Rosiak, M. Rössle, Synthesis 2007, 1279;
- 13bT. Hayashi, Bull. Chem. Soc. Jpn. 2004, 77, 13;
- 13cT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829;
- 13dK. Fagnou, M. Lautens, Chem. Rev. 2003, 103, 169;
- 13eC. Bolm, J. P. Hildebrand, K. Muñiz, N. Hermanns, Angew. Chem. 2001, 113, 3382;
Angew. Chem. Int. Ed. 2001, 40, 3284.
10.1002/1521-3773(20010917)40:18<3284::AID-ANIE3284>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 14Insertion of an alkyne to arylrhodium species that have a diene ligand is known to be more facile than that of an electron-deficient olefin. See Ref. [9g].
- 15For examples of rhodium-catalyzed asymmetric 1,4-additions to create quaternary carbon stereocenters, see:
- 15aP. Mauleón, J. C. Carretero, Chem. Commun. 2005, 4961;
- 15bR. Shintani, W.-L. Duan, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 5628;
- 15cR. Shintani, Y. Tsutsumi, M. Nagaosa, T. Nishimura, T. Hayashi, J. Am. Chem. Soc. 2009, 131, 13588.
- 16For examples of copper catalysis, see:
- 16aJ. Wu, D. M. Mampreian, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 4584;
- 16bE. Fillion, A. Wilsily, J. Am. Chem. Soc. 2006, 128, 2774;
- 16cD. Martin, S. Kehrli, M. d’Augustin, H. Clavier, M. Mauduit, A. Alexakis, J. Am. Chem. Soc. 2006, 128, 8416;
- 16dM. d’Augustin, L. Palais, A. Alexakis, Angew. Chem. 2005, 117, 1400;
10.1002/ange.200462137 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1376;
- 16eT. L. May, M. K. Brown, A. H. Hoveyda, Angew. Chem. 2008, 120, 7468; Angew. Chem. Int. Ed. 2008, 47, 7358;
- 16fK. M. Brown, A. H. Hoveyda, J. Am. Chem. Soc. 2008, 130, 12904.
- 17For reviews on chiral dienes, see:
- 17aR. Shintani, T. Hayashi, Aldrichimica Acta 2009, 42, 31;
- 17bC. Defieber, H. Grützmacher, E. M. Carreira, Angew. Chem. 2008, 120, 4558;
10.1002/ange.200703612 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4482.
- 18For examples of asymmetric reactions using chiral diene ligands, see:
- 18aC. Fischer, C. Defieber, T. Suzuki, E. M. Carreira, J. Am. Chem. Soc. 2004, 126, 1628;
- 18bJ.-F. Paquin, C. Defieber, C. R. J. Stephenson, E. M. Carreira, J. Am. Chem. Soc. 2005, 127, 10850;
- 18cF. Läng, F. Breher, D. Stein, H. Grützmacher, Organometallics 2005, 24, 2997;
- 18dS. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331;
- 18eZ.-Q. Wang, C.-G. Feng, M.-H. Xu, G.-Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336;
- 18fT. Noël, K. Vandyck, J. Van der Eycken, Tetrahedron 2007, 63, 12961;
- 18gT. Gendrineau, O. Chuzel, H. Eijsberg, J.-P. Genet, S. Darses, Angew. Chem. 2008, 120, 7783;
10.1002/ange.200803230 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7669;
- 18hX. Hu, M. Zhuang, Z. Cao, H. Du, Org. Lett. 2009, 11, 4744;
- 18iM. K. Brwon, E. J. Corey, Org. Lett. 2010, 12, 172.
- 19
- 19aT. Hayashi, K. Ueyama, N. Tokunaga, K. Yoshida, J. Am. Chem. Soc. 2003, 125, 11508;
- 19bY. Otomaru, A. Kina, R. Shintani, T. Hayashi, Tetrahedron: Asymmetry 2005, 16, 1673;
- 19cA. Kina, K. Ueyama, T. Hayashi, Org. Lett. 2005, 7, 5889;
- 19dR. Shintani, Y. Ichikawa, K. Takatsu, F.-X. Chen, T. Hayashi, J. Org. Chem. 2009, 74, 869;
- 19eT. Nishimura, H. Kumamoto, M. Nagaosa, T. Hayashi, Chem. Commun. 2009, 5713.
- 20K. Okamoto, T. Hayashi, V. H. Rawal, Chem. Commun. 2009, 4815.
- 21
- 21aN. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2004, 126, 13584;
- 21bY. Otomaru, K. Okamoto, R. Shintani, T. Hayashi, J. Org. Chem. 2005, 70, 2503.
- 22D. Yang, C. Zhang, J. Org. Chem. 2001, 66, 4814.
- 23CCDC 764834 (4) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.