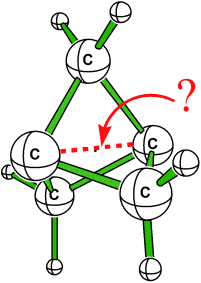
The Inverted Bond in [1.1.1]Propellane is a Charge-Shift Bond†
Wei Wu Prof.
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorJunjing Gu
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorJinshuai Song
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorSason Shaik Prof.
Institute of Chemistry and The Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University, Jerusalem, 91904 (Israel), Fax: (+972) 2-658-4680
Search for more papers by this authorPhilippe C. Hiberty Prof.
Laboratoire de Chimie Physique, Groupe de Chimie Théorique, CNRS UMR 8000, Université de Paris-Sud, 91405 Orsay Cédex (France), Fax: (+33) 1-6941-6175
Search for more papers by this authorWei Wu Prof.
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorJunjing Gu
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorJinshuai Song
State Key Laboratory of Physical Chemistry of Solid Surfaces and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005 (P.R. China), Fax: (+86) 592-218-4708
Search for more papers by this authorSason Shaik Prof.
Institute of Chemistry and The Lise Meitner-Minerva Center for Computational Quantum Chemistry, The Hebrew University, Jerusalem, 91904 (Israel), Fax: (+972) 2-658-4680
Search for more papers by this authorPhilippe C. Hiberty Prof.
Laboratoire de Chimie Physique, Groupe de Chimie Théorique, CNRS UMR 8000, Université de Paris-Sud, 91405 Orsay Cédex (France), Fax: (+33) 1-6941-6175
Search for more papers by this authorThe research in Xiamen is supported by the Natural Science Foundation of China (No 20533020, 20873106) and the National Basic Research Program of China (204CB719902). S.S. is supported by an ISF grant (16/06)
Abstract
Gebunden, aber wie? Eine Ab-initio-Studie von [1.1.1]Propellan spricht für eine starke σ-Bindung zwischen den beiden Brückenkopf-Kohlenstoffatomen, die aber weder klassisch kovalent noch klassisch ionisch, sondern eine Ladungsverschiebungsbindung ist, in der die Resonanzenergie zwischen kovalenten und ionischen Strukturen die Hauptrolle spielt. Insofern spiegelt die zentrale Bindung in [1.1.1]Propellan die Einfachbindung in Difluor gut wider.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| ange_200804965_sm_miscellaneous_information.pdf272.2 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. D. Newton, J. M. Schulman, J. Am. Chem. Soc. 1972, 94, 773.
- 2K. B. Wiberg, F. H. Walker, J. Am. Chem. Soc. 1982, 104, 5239.
- 3J. E. Jackson, L. C. Allen, J. Am. Chem. Soc. 1984, 106, 591.
- 4K. B. Wiberg, W. P. Dailey, F. H. Walker, S. T. Waddell, L. S. Crocker, M. D. Newton, J. Am. Chem. Soc. 1985, 107, 7247.
- 5D. Feller, E. R. Davidson, J. Am. Chem. Soc. 1987, 109, 4133.
- 6
- 6aR. P. Messmer, P. A. Schultz, J. Am. Chem. Soc. 1986, 108, 7407;
- 6bR. M. Jarret, L. Cucumano, Tetrahedron Lett. 1990, 31, 171;
- 6cA. Ebrahimi, F. Deyhimi, H. Roohi, J. Mol. Struct. (Theochem) 2003, 626, 223;
- 6dM. Pecul, H. Dodziuk, M. Jaszunski, O. Lukin, J. Leszczynski, Phys. Chem. Chem. Phys. 2001, 3, 1986;
- 6eL. B. Krivdin, Magn. Reson. Chem. 2004, 42, 1.
- 7K. B. Wiberg, J. Am. Chem. Soc. 1983, 105, 1227.
- 8K. B. Wiberg, R. F. W. Bader, C. D. H. Lau, J. Am. Chem. Soc. 1987, 109, 985.
- 9K. B. Wiberg, Chem. Rev. 1989, 89, 975.
- 10
- 10aP. Chakrabarti, P. Seiler, J. D. Dunitz, A.-D. Schluter, G. Szeimies, J. Am. Chem. Soc. 1981, 103, 7378;
- 10bP. Seiler, J. Belzner, G. Szeimies, Helv. Chim. Acta 1988, 71, 2100.
- 11M. Messerschmidt, S. Scheins, L. Grubert, M. Pätzel, G. Szeimies, C. Paulmann, P. Luger, Angew. Chem. 2005, 117, 3993;
10.1002/ange.200500169 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3925.
- 12For example, the Laplacian of the density at the BCP amounts to 0.58 for the CS bond of F2, versus −0.62 for the classical CC bond of ethane. See: L. Zhang, F. Ying, W. Wu, P. C. Hiberty, S. Shaik, Chem. Eur. J. 2008, in press.
- 13
- 13aS. Shaik, P. Maître, G. Sini, P. C. Hiberty, J. Am. Chem. Soc. 1992, 114, 7861;
- 13bA. Shurki, P. C. Hiberty, S. Shaik, J. Am. Chem. Soc. 1999, 121, 822;
- 13cP. C. Hiberty, C. Megret, L. Song, W. Wu, S. Shaik, J. Am. Chem. Soc. 2006, 128, 2836;
- 13dP. C. Hiberty, R. Ramozzi, W. Wu, S. Shaik, Faraday Discuss. 2007, 135, 261.
- 14S. Shaik, D. Danovich, B. Silvi, D. Lauvergnat, P. C. Hiberty, Chem. Eur. J. 2005, 11, 6358.
- 15V. Polo, J. Andres, B. Silvi, J. Comput. Chem. 2007, 28, 857.
- 16R. LLusar, A. Bertran, J. Andres, S. Noury, B. Silvi, J. Comput. Chem. 1999, 20, 1517.
- 17We are thankful to one of the referees for inducing us to make this prediction.
- 18
- 18aP. C. Hiberty, J. P. Flament, E. Noizet, Chem. Phys. Lett. 1992, 189, 259;
- 18bP. C. Hiberty, S. Humbel, C. P. Byrman, J. H. van Lenthe, J. Chem. Phys. 1994, 101, 5969;
- 18cP. C. Hiberty, S. Shaik, Theor. Chem. Acc. 2002, 108, 255.
- 19M. J. Frisch et al., Gaussian 03, Revision D.01; Gaussian, Inc.: Wallingford CT, 2004 (see Supporting Information).
- 20H.-J. Werner et al., MOLPRO, a package of ab initio programs, Version 2002.1 (see Supporting Information).
- 21
- 21aL. Song, Y. Mo, Q. Zhang, W. Wu, XMVB: An Ab Initio Nonorthogonal Valence Bond Program, Xiamen University, Xiamen, 1999;
- 21bL. Song, Y. Mo, Q. Zhang, W. Wu, J. Comput. Chem. 2005, 26, 514.
- 22For example, a pentagerma[1.1.1]propellane has been synthesized: See D. Nied, W. Klopper, F. Breher, Angew. Chem. 2009, 121, 1439; Angew. Chem. Int. Ed. 2009, 48, 1411.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.