Time-Controlled Microfluidic Seeding in nL-Volume Droplets To Separate Nucleation and Growth Stages of Protein Crystallization†
This work was supported by NIH Protein Structure Initiative Specialized Centers Grant GM074961 (ATCG3D) and the NIH (R01 EB001903). Use of the Advanced Photon Source was supported by the US Department of Energy (contract no. W-31–109-Eng-38). Use of the BioCARS Sector 14 was supported by the NIH National Center for Research Resources (grant number RR07707). GM/CA-CAT has been funded in whole or in part by the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Sciences (Y1-GM-1104). Funding for functional and structural proteomics of SARS CoV-related proteins is provided through NIH-NIAID contract HHSN266200400058C. We thank Ruslan Sanishvili (GM/CA Cat station 23ID-D staff support) for technical assistance; Scott Lovell and Lance Stewart (deCODE Biostructures) for helpful assistance and discussions; Shu Moy (Midwest Center for Structural Genomics) for cloning work on Oligoendopeptidase F; Vanitha Subramanian (The Scripps Research Institute) for cloning, expression, and purification of SARS nucleocapsid N-terminal domain; and L. Spencer Roach (University of Chicago) for help with thaumatin X-ray diffraction comparisons.
Graphical Abstract
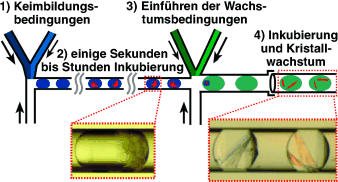
Aussaat: Um geordnete Proteinkristalle zu erhalten, müssen die beiden Schlüsselschritte der Kristallisation, Keimbildung und Wachstum, kontrolliert werden. Ihre Idealbedingungen sind jedoch oft unterschiedlich. Mit einem Mikrofluidiksystem (siehe Bild) gelang das zeitgesteuerte „Säen“ von Kristallen in nL-Volumina. Einkristalle des Proteins Oligoendopeptidase F wurden erhalten und für die Röntgenstrukturanalyse genutzt.