“Hydrogen-Catalyzed” Dehydrogenation: A Supercritical Conundrum†
Jason R. Hyde Dr.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorBen Walsh Dr.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorMartyn Poliakoff Prof.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorJason R. Hyde Dr.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorBen Walsh Dr.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorMartyn Poliakoff Prof.
School of Chemistry, University of Nottingham, University Park, NG7 2RD, UK, Fax: (+44) 115-913-058
Search for more papers by this authorWe thank the EPSRC for Grant GR/S87409 and for a CASE studentship to B.W. and Thomas Swan & Co. Ltd for support. We thank Dr. Pete Licence, Dr. Stephen Ross, and Messrs Mark Guyler, Peter Fields, Rich Wilson, David Litchfield, and James Warren for their help and support.
Graphical Abstract
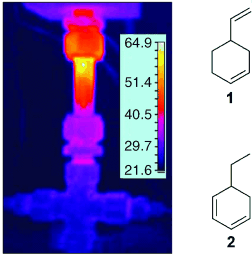
Hot Spots: Die überraschende, offenbar katalytische Wirkung von H2 bei der Dehydrierung von 1 in überkritischem CO2 ist das Ergebnis thermischer Hot Spots (siehe Thermobild des Reaktors), die zu Beginn im Katalysatorbett durch eine exotherme Hydrierung erzeugt werden. Dieses punktuelle Erhitzen erzwingt die Dehydrierung und ermöglicht einen einfachen Zugang zu Dien 2.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2005/z502049_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. R. Hyde, M. Poliakoff, R. Amandi, S. K. Ross, T. J. Lotz, Green Chem. 2005, 7, 288.
- 2J. M. Simard, M. S. Erickson, Abstr. Pap. Am. Chem. Soc. 1999, 217, 271.
- 3M. G. Hitzler, F. R. Smail, S. K. Ross, M. Poliakoff, Chem. Commun. 1998, 359.
- 4J. E. Chateauneuf, K. Nie, Abstr. Pap. Am. Chem. Soc. 2000, 219, 446.
- 5W. Leitner, Pure Appl. Chem. 2004, 76, 635.
- 6A. R. Tadd, A. Marteel, M. R. Mason, J. A. Davies, M. A. Abraham, J. Supercrit. Fluids 2003, 25, 183.
- 7A. Marteel, J. A. Davies, M. R. Mason, T. Tack, S. Bektesevic, M. A. Abraham, Catal. Commun. 2003, 4, 309.
- 8A. E. Marteel, T. T. Tack, S. Bektesevic, J. A. Davies, M. R. Mason, M. A. Abraham, Environ. Sci. Technol. 2003, 37, 5424.
- 9N. J. Meehan, A. J. Sandee, J. N. H. Reek, P. C. J. Kamer, P. van Leeuwen, M. Poliakoff, Chem. Commun. 2000, 1497.
- 10M. Caravati, J. D. Grunwaldt, A. Baiker, Catal. Today, 2004, 91–92, 1.
- 11T. D. Crowe, P. J. White, J. Am. Oil Chem. Soc. 2003, 80, 569.
- 12M. L. Corazza, L. Cardozo, O. A. C. Antunes, C. Dariva, Ind. Eng. Chem. Res. 2003, 42, 3150.
- 13J. Liu, R. Zhang, L. Gao, T. Jiang, Z. M. Liu, J. He, B. X. Han, G. Y. Yang, Chin. Chem. Lett. 2002, 13, 1162.
- 14G. T. Musie, M. Wei, B. Subramaniam, D. H. Busch, Inorg. Chem. 2001, 40, 3336.
- 15G. Jenzer, M. S. Schneider, R. Wandeler, T. Mallat, A. Baiker, J. Catal. 2001, 199, 141.
- 16J. R. Hyde, M. Poliakoff, B. Walsh, Green Chem. 2005, 7, 357.
- 17W. K. Gray, F. R. Smail, M. G. Hitzler, S. K. Ross, M. Poliakoff, J. Am. Chem. Soc. 1999, 121, 10711.
- 18P. Licence, W. K. Gray, M. Sokolova, J. Am. Chem. Soc. 2005, 127, 293.
- 19S. Ichikawa, M. Tada, Y. Iwasawea, T. Ikariya, Chem. Commun. 2005, 924.
- 20P. Stephenson, P. Licence, S. K. Ross, M. Poliakoff, Green Chem. 2004, 6, 521.
- 21F. Zhao, Y. Ikushima, M. Arai, J. Catal. 2004, 224, 479.
- 22F. Y. Zhao, S. Fujita, J. M. Sun, Y. Ikushima, M. Arai, Catal. Today 2004, 98, 523.
- 23T. Sato, C. V. Rode, O. Sato, M. Shirai, Appl. Catal. B 2004, 49, 181.
- 24N. Hiyoshi, C. V. Rode, O. Sato, M. Shirai, J. Jpn. Pet. Inst. 2004, 47, 410.
- 25J. R. Hyde, P. Licence, D. Carter, M. Poliakoff, Appl. Catal. A 2001, 222, 119.
- 26S. K. Ross, F. R. Smail, M. Sellin, R. Amandi, M. Poliakoff, Chem. Eng. Trans. 2002, 2, 561.
- 27S. Clark, US Patent 5 321 180, 1994.
- 28R. W. Rieve, H. Shalit, US Patent 4 029 715, 1977.
- 29G. Ruckelshauss, US Patent 4 048 243, 1997.
- 30H. H. Vogel, H. M. Weitz, E. Lorez, R. Platz, US Patent 3 903 185, 1975.
- 31D. W. Hart, L. H. Slaugh, US Patent 4 375 571, 1983.
- 32O. Levenspiel, 1st ed., Chemical Reaction Engineering, 2nd Ed. 1962, 230.
- 33The residence time was estimated by calculating the velocity of the fluid passing through the reactor per unit volume. Fluid velocity (m s−1) was calculated at experimental temperature and pressure, and therefore requires specific data, which was obtained from appropriate equations of state, in this case from the NIST database (http://www.nist.gov).
- 34J. R. Hyde, R. A. Bourne, I. Noda, P. Stephenson, M. Poliakoff, Anal. Chem. 2004, 76, 6197.
- 35Advanced Chemistry Development, Inc., 6.08, Toronto ON, Canada, http://www.acdlabs.com, 2002.
- 36J. H. Kim, D. J. Suh, T. J. Park, K. L. Kim, Appl. Catal. A 2000, 197, 191.
- 37B. Trost, T. Runge, L. Jungheim, J. Am. Chem. Soc. 1980, 102, 2841.
- 38I. Noda, Appl. Spectrosc. 1993, 47, 1329.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.