The Origin of Solvent-Controlled Supramolecular Chirality Switching in a Bis(Zinc Porphyrin) System
Victor V. Borovkov Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorGuy A. Hembury Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorYoshihisa Inoue Prof. Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorVictor V. Borovkov Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorGuy A. Hembury Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorYoshihisa Inoue Prof. Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorGraphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z52493_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. S. Balaban, A. D. Bhise, M. Fischer, M. Linke-Schaetzel, C. Roussel, N. Vanthuyne, Angew. Chem. 2003, 115, 2190–2194;
10.1002/ange.200250465 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2140–2144;
- 1bV. V. Borovkov, T. Harada, G. A. Hembury, Y. Inoue, R. Kuroda, Angew. Chem. 2003, 115, 1788–1791;
10.1002/ange.200250524 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1746–1749;
- 1cM. Ishikawa, K. Maeda, E. Yashima, J. Am. Chem. Soc. 2002, 124, 7448–7458;
- 1dT. Nabeshima, A. Hashiguchi, T. Saiki, S. Akine, Angew. Chem. 2002, 114, 499–502;
10.1002/1521-3757(20020201)114:3<499::AID-ANGE499>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 2002, 41, 481–484;10.1002/1521-3773(20020201)41:3<481::AID-ANIE481>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 1eM. Ribó, J. Crusats, F. Sagués, J. Claret, R. Rubires, Science 2001, 292, 2063–2066;
- 1fH. Nakashima, J. R. Koe, K. Torimitsu, M. Fujiki, J. Am. Chem. Soc. 2001, 123, 4847–4849;
- 1gJ. M. Fox, T. J. Katz, S. Van Elshocht, T. Verbiest, M. Kauranen, A. Persoons, T. Thongpanchang, T. Kraus, L. Brus, J. Am. Chem. Soc. 1999, 121, 3453–3459;
- 1hR. Purrello, A. Raudino, L. M. Scolaro, A. Loisi, E. Bellacchio, R. Lauceri, J. Phys. Chem. B 2000, 104, 10 900–10 908;
- 1iD. B. Steensgaard, H. Wackerbarth, P. Hildebrandt, A. R. Holzwarth, J. Phys. Chem. B 2000, 104, 10 379–10 386;
- 1jD. Iarossi, A. Mucci, F. Parenti, L. Schenetti, R. Seeber, C. Zanardi, A. Forni, M. Tonelli, Chem. Eur. J. 2001, 7, 676–685;
10.1002/1521-3765(20010202)7:3<676::AID-CHEM676>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 1kM. de Loos, J. van Esch, R. M. Kellogg, B. L. Feringa, Angew. Chem. 2001, 113, 633–636;
10.1002/1521-3757(20010202)113:3<633::AID-ANGE633>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2001, 40, 613–616;10.1002/1521-3773(20010202)40:3<613::AID-ANIE613>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 1lW. Steffen, B. Kohler, M. Altmann, U. Scherf, K. Stitzer, H.-C. zur Loye, U. H. F. Bunz, Chem. Eur. J. 2001, 7, 117–126;
10.1002/1521-3765(20010105)7:1<117::AID-CHEM117>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 1mL. Brunsveld, E. W. Meijer, R. B. Prince, J. S. Moore, J. Am. Chem. Soc. 2001, 123, 7978–7984;
- 1nJ. H. Jung, H. Kobayashi, M. Masuda, T. Shimizu, S. Shinkai, J. Am. Chem. Soc. 2001, 123, 8785–8789;
- 1oM. Wang, G. L. Silva, B. A. Armitage, J. Am. Chem. Soc. 2000, 122, 9977–9986;
- 1pP. Wittung, P. Nielsen, O. Buchart, M. Egholm, B. Norden, Nature 1994, 368, 561–563.
- 2
- 2aT. Aoki, T. Kaneko, N. Maruyama, A. Sumi, M. Takahashi, T. Sato, M. Teraguchi, J. Am. Chem. Soc. 2003, 125, 6346–6347;
- 2bT. Kawasaki, M. Tokuhiro, N. Kimizuka, T. Kunitake, J. Am. Chem. Soc. 2002, 124, 11 282–11 283;
- 2cH. Nakashima, M. Fujiki, J. R. Koe, M. Motonaga, J. Am. Chem. Soc. 2001, 123, 1963–1969;
- 2dF. X. Redl, M. Lutz, J. Daub, Chem. Eur. J. 2001, 7, 5350–5358;
10.1002/1521-3765(20011217)7:24<5350::AID-CHEM5350>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 2eT. Kurtan, N. Nesnas, Y.-Q. Li, X. Huang, K. Nakanishi, N. Berova, J. Am. Chem. Soc. 2001, 123, 5962–5973;
- 2fK. Tomizaki, H. Nishino, T. Kato, A. Miike, N. Nishino, Chem. Lett. 2000, 648–649;
- 2gS. E. Boiadjiev, D. Lightner, J. Am. Chem. Soc. 2000, 122, 378–383;
- 2hS. Yagi, T. Morinaga, T. Nomura, T. Takagishi, T. Mizutani, S. Kitagawa, H. Ogoshi, J. Org. Chem. 2001, 66, 3848–3853;
- 2iJ. Recker, D. J. Tomcik, J. R. Parquette, J. Am. Chem. Soc. 2000, 122, 10 298–10 307;
- 2jS. Saito, C. Nuckolls, J. Rebek, Jr., J. Am. Chem. Soc. 2000, 122, 9628–9630.
- 3
- 3aV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, Helv. Chim. Acta 1999, 82, 919–934.
10.1002/(SICI)1522-2675(19990609)82:6<919::AID-HLCA919>3.0.CO;2-P CAS Web of Science® Google Scholar
- 4
- 4aV. V. Borovkov, T. Harada, Y. Inoue, R. Kuroda, Angew. Chem. 2002, 114, 1436–1439;
10.1002/1521-3757(20020415)114:8<1436::AID-ANGE1436>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1378–1381;10.1002/1521-3773(20020415)41:8<1378::AID-ANIE1378>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 4bV. V. Borovkov, J. M. Lintuluoto, M. Sugiura, Y. Inoue, R. Kuroda, J. Am. Chem. Soc. 2002, 124, 11 282–11 283;
- 4cV. V. Borovkov, J. M. Lintuluoto, H. Sugeta, M. Fujiki, R. Arakawa, Y. Inoue, J. Am. Chem. Soc. 2002, 124, 2993–3006;
- 4dV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, J. Am. Chem. Soc. 2001, 123, 2979–2989;
- 4eV. V. Borovkov, N. Yamamoto, J. M. Lintuluoto, T. Tanaka, Y. Inoue, Chirality 2001, 13, 329–335;
- 4fV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, Org. Lett. 2002, 4, 169–171;
- 4gV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, Org. Lett. 2000, 2, 1565–1568;
- 4hV. V. Borovkov, J. M. Lintuluoto, M. Fujiki, Y. Inoue, J. Am. Chem. Soc. 2000, 122, 4403–4407;
- 4iV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, J. Phys. Chem. A 2000, 104, 9213–9219;
- 4jV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, J. Phys. Chem. B 1999, 103, 5151–5156.
- 5The term induced circular dichroism (ICD) was originally proposed by Prof. H. Falk for the phenomenon of asymmetry transfer from chiral solvents to achiral chromophores (for details, see H. Falk, W. Jungwirth, N. Müller, Monatsh. Chem. 1984, 115, 455–466).
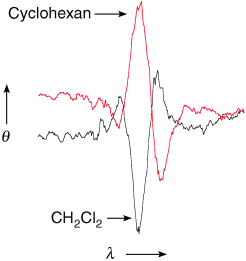
- 6The choice of solvents (see Supporting Information) was limited to those that do not display any coordination to zinc porphyrins.
- 7M. Kasha, H. R. Rawls, M. A. El-Bayoumi, Pure Appl. Chem. 1965, 11, 371–392.
- 8(CH2Cl)2 is an exceptional case among the polar solvents studied with enhancement of the B2 transition over B1 transition, apparently because of its inability to form the corresponding solvation shell owing to steric hindrance.
- 9N. Harada, K. Nakanishi, Circular Dichroic Spectroscopy. Exciton Coupling in Organic Stereochemistry, University Science Books, Mill Valley, CA, 1983.
- 10M. Nishio, M. Hirota, Y. Umezawa, The CH/π Interaction: Evidence, Nature, and Consequence, Wiley-VCH, New York, 1998.
- 11In the case of 1⋅(3)2 the extremely narrow εs gap of chirality switching is apparently because benzene also enhances a positive component of chirality induction for the same reasons as in the case of 1⋅42 and 1⋅52 but to a lesser extent.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.