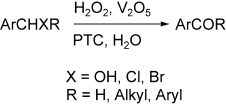The Dual Roles of Oxodiperoxovanadate Both as a Nucleophile and an Oxidant in the Green Oxidation of Benzyl Alcohols or Benzyl Halides to Aldehydes and Ketones
Correction(s) for this article
-
The Dual Roles of Oxodiperoxovanadate Both as a Nucleophile and an Oxidant in the Green Oxidation of Benzyl Alcohols or Benzyl Halides to Aldehydes and Ketones
- Chunbao Li Dr.,
- Pengwu Zheng,
- Jie Li,
- Hang Zhang,
- Yi Cui,
- Qiyun Shao,
- Xiujie Ji,
- Jian Zhang,
- Pengying Zhao,
- Yanli Xu,
- Volume 119Issue 30Angewandte Chemie
- pages: 5744-5744
- First Published online: July 13, 2007
Chunbao Li Dr.
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorPengwu Zheng
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorJie Li
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorHang Zhang
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorYi Cui
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorQiyun Shao
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorXiujie Ji
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorJian Zhang
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorPengying Zhao
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorYanli Xu
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorChunbao Li Dr.
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorPengwu Zheng
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorJie Li
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorHang Zhang
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorYi Cui
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorQiyun Shao
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorXiujie Ji
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorJian Zhang
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorPengying Zhao
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorYanli Xu
Department of Chemistry, School of Sciences, Tianjin University, Tianjin 300072, China, Fax: (+86) 22-2740-3475
Search for more papers by this authorGraphical Abstract
Doppelrolle: Oxodiperoxovanadat (VO(O2)2−) reagiert unter sauren Bedingungen als Nucleophil und als Oxidationsmittel für Benzylalkohole und -halogenide. Diese werden mit H2O2 in Wasser mit VO(O2)2− als Katalysator in Gegenwart eines Phasentransferkatalysators (PTC) zu aromatischen Aldehyden und Ketonen umgesetzt (siehe Schema).
References
- 1C. Li, Y. Xu, M. Lu, Z. Zhao, L. Liu, Z. Zhao, Y. Cui, P. Zheng, X. Ji, G. Gao, Synlett 2002, 2041.
- 2R. C. Larock, Comprehensive Organic Transformations, Vol. 1, Wiley, New York, 1999, pp. 1205–1261.
- 3M. Hudlicky, Oxidations in Organic Chemistry, American Chemical Society, Woshington, DC, 1990, pp. 114–159.
- 4K. Sato, M. Aoki, J. Takagi, K. Zimmermann, R. Noyori, Bull. Chem. Soc. Jpn. 1999, 72, 2287.
- 5A. Butler, M. J. Clague, G. E. Meister, Chem. Rev. 1994, 94, 625.
- 6O. Bortolini, V. Conte, F. Di Furia, G. Modena, Nouv. J. Chim. 1985, 9, 147.
- 7R. Neumann, M. Levin-Elad, Appl. Catal. A 1995, 122, 85.
- 8Y. Maeda, N. Kakiuchi, S. Matsumura. T. Nishimura. T. Kawamura, S. Uemura, J. Org. Chem. 2002, 67, 6718.
- 9R. Wever, M. B. E. Krenn in Vanadium in Biological System (Ed.: ), Kluwer Academic Publishers, Dordrecht, The Netherlands, 1990, p. 81.
10.1007/978-94-009-2023-1_5 Google Scholar
- 10U. Bora, G. Bose. M. K. Chaudhurl, S. S. Dahr, R. Gopinath, A. T. Khan, B. K. Patel, Org. Lett. 2000, 2, 247.
- 11H. Kelm, H.-J. Krueger, Inorg. Chem. 1996, 35, 3553.
- 12G. J. Colpas, B. J. Hamstra, J. W. Kampf, V. L. Pecoraro, J. Am. Chem. Soc. 1996, 118, 3469.
- 13Ullmann's Encyclopedia of Industrial Chemistry, 7th ed., Electronic release, Wiley, VCH, 2003.
- 14R. A. Sheldon, I. W. C. E. Arends, A, Dijksman, Catal. Today 2000, 57, 157.
- 15R. C. Larock, Comprehensive Organic Transformations, Vol. 2, Wiley, New York, 1999, pp. 1222–1225.
- 16T. Nishimura, T. Onoue, K. Ohe, S. Uemura, J. Org. Chem. 1999, 64, 6750.
- 17N. Kornblum, W. J. Jones, G. J. Anderson, J. Am. Chem. Soc. 1959, 81, 4113.
- 18M. G. Edwards, J. M. J. Williams, Angew. Chem. 2002, 114, 4934;
10.1002/ange.200290033 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4740.
- 19A. Itoh, T. Kodana, S. Inagaki, Y. Masaki, Org. Lett. 2000, 2, 2455.
- 20H. B. Hass, M. L. Bender, J. Am. Chem. Soc. 1949, 71, 1767.
- 21V. Conte, F. Di Furia, G. Modena, J. Org. Chem. 1988, 53, 1665.
- 22F. Kröhnke, Angew. Chem. 1963, 75, 317; Angew. Chem. Int. Ed. Engl. 1963, 2, 380.
- 23
- 23aH. Ji, T. Mizugaki, K. Ebitani, K. Kaneda, Tetrahedron Lett. 2002, 43, 7179;
- 23bK. Yamaguchi, N. Mizuno, Angew. Chem. 2002, 114, 4720;
10.1002/1521-3757(20021202)114:23<4720::AID-ANGE4720>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4538.10.1002/1521-3773(20021202)41:23<4538::AID-ANIE4538>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 24S. Suzuki, T. Onishi, Y. Fujita, H. Misawa, J. Otera, Bull. Chem. Soc. Jpn. 1986, 59, 3287.
- 25C.-G. Jia, F.-Y. Jing, W.-D. Hu, M.-Y. Huang, Y.-Y. Jiang, J. Mol. Catal. 1994, 91, 139.
- 26S. Das, A. K. Panigrahi, G. C. Maikap, Tetrahedron Lett. 2003, 44, 1375.
- 27J. Martin, C. Martin, M. Faraj, J.-M. Bregeault, Nouv. J. Chim. 1984, 8, 141.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.