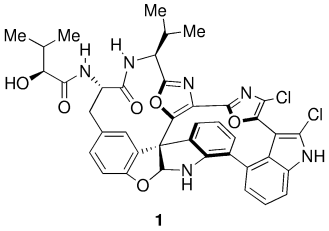
The Second Total Synthesis of Diazonamide A†
K. C. Nicolaou Prof. Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorParaselli Bheema Rao Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorJunliang Hao Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorMali Venkat Reddy Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorGerasimos Rassias Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorXianhai Huang Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorDavid Y.-K. Chen Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorScott A. Snyder
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorK. C. Nicolaou Prof. Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorParaselli Bheema Rao Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorJunliang Hao Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorMali Venkat Reddy Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorGerasimos Rassias Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorXianhai Huang Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorDavid Y.-K. Chen Dr.
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorScott A. Snyder
Department of Chemistry and, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA, Fax: (+1) 858-784-2469
Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA
Search for more papers by this authorWe thank Dr. D. H. Huang and Dr. G. Siuzdak for NMR spectroscopic and mass spectrometric assistance, respectively. Financial support for this work was provided by The Skaggs Institute for Chemical Biology, American Biosciences, the National Institutes of Health (USA), predoctoral fellowships from the National Science Foundation and Pfizer (S.A.S.), and a postdoctoral fellowship from The Skaggs Institute for Chemical Biology (X.H.).
Graphical Abstract
Die hoch gespannte einzigartige Molekülstruktur und die potenzielle tumortherapeutische Wirkung von Diazonamid A (1) machen diesen Naturstoff zu einem attraktiven Syntheseziel. Zu den Schlüsselschritten dieser Totalsynthese von 1 zählen die neuartige SmI2-gestützte Ringöffnungs-Sequenz und die ungewöhnliche Oxidation eines Indolins zu einem Oxindol in Gegenwart von Pd(OH)2/C.
References
- 1For discussions of this topic in the context of total synthesis, see examples in the following:
- 1aK. C. Nicolaou, D. Vourloumis, N. Winssinger, P. S. Baran, Angew. Chem. 2000, 112, 46–126;
10.1002/(SICI)1521-3757(20000103)112:1<46::AID-ANGE46>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2000, 39, 44–122;10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 1bK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. A. Vassilikogiannakis, Angew. Chem. 2002, 114, 1742–1773;
10.1002/1521-3757(20020517)114:10<1742::AID-ANGE1742>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1668–1698;10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 1cK. C. Nicolaou, P. S. Baran, Angew. Chem. 2002, 114, 2800–2843;
10.1002/1521-3757(20020802)114:15<2800::AID-ANGE2800>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2678–2720.10.1002/1521-3773(20020802)41:15<2678::AID-ANIE2678>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 2For the isolation and original structural assignments, see:
- 2aN. Lindquist, W. Fenical, G. D. Van Duyne, J. Clardy, J. Am. Chem. Soc. 1991, 113, 2303–2304; for the synthesis of the originally proposed structure and revision of the structure of diazonamide A, see:
- 2bJ. Li, S. Jeong, L. Esser, P. G. Harran, Angew. Chem. 2001, 113, 4901–4906;
Angew. Chem. Int. Ed. 2001, 40, 4765–4770;
10.1002/1521-3773(20011217)40:24<4765::AID-ANIE4765>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 2cJ. Li, A. W. G. Burgett, L. Esser, C. Amezcua, P. G. Harran, Angew. Chem. 2001, 113, 4906–4909;
Angew. Chem. Int. Ed. 2001, 40, 4770–4773.
10.1002/1521-3773(20011217)40:24<4770::AID-ANIE4770>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 3For highlights of previous synthetic studies towards the diazonamides, see:
- 3aV. Wittmann, Nachr. Chem. 2002, 50, 477–482;
- 3bT. Ritter, E. M. Carreira, Angew. Chem. 2002, 114, 2601–2606;
Angew. Chem. Int. Ed. 2002, 41, 2489–2495.
10.1002/1521-3773(20020715)41:14<2489::AID-ANIE2489>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 4aJ. Li, X. Chen, A. W. G. Burgett, P. G. Harran, Angew. Chem. 2001, 113, 2754–2757;
Angew. Chem. Int. Ed. 2001, 40, 2682–2685;
10.1002/1521-3773(20010716)40:14<2682::AID-ANIE2682>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 4bX. Chen, L. Esser, P. G. Harran, Angew. Chem. 2000, 112, 967–970;
Angew. Chem. Int. Ed. 2000, 39, 937–940;
10.1002/(SICI)1521-3773(20000303)39:5<937::AID-ANIE937>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 4cS. Jeong, X. Chen, P. G. Harran, J. Org. Chem. 1998, 63, 8640–8641.
- 5aE. Vedejs, M. A. Zajac, Org. Lett. 2001, 3, 2451–2454;
- 5bE. Vedejs, J. Wang, Org. Lett. 2000, 2, 1031–1032;
- 5cE. Vedejs, D. A. Barba, Org. Lett. 2000, 2, 1033–1035.
- 6aP. Wipf, J.-L. Methot, Org. Lett. 2001, 3, 1261–1264;
- 6bP. Wipf, F. Yokokawa, Tetrahedron Lett. 1998, 39, 2223–2226.
- 7aJ. D. Kreisberg, P. Magnus, E. G. McIver, Tetrahedron Lett. 2001, 42, 627–629;
- 7bP. Magnus, E. G. McIver, Tetrahedron Lett. 2000, 41, 831–834;
- 7cF. Chan, P. Magnus, E. G. McIver, Tetrahedron Lett. 2000, 41, 835–838;
- 7dP. Magnus, J. D. Kreisberg, Tetrahedron Lett. 1999, 40, 451–454.
- 8D. E. Fuerst, B. M. Stoltz, J. L. Wood, Org. Lett. 2000, 2, 3521–3523.
- 9aS. Schley, A. Radspieler, G. Christoph, J. Liebscher, Eur. J. Org. Chem. 2002, 369–374;
- 9bA. Radspieler, J. Liebscher, Synthesis 2001, 745–750.
- 10aF. Lach, C. J. Moody, Tetrahedron Lett. 2000, 41, 6893–6896;
- 10bM. C. Bagley, S. L. Hind, C. J. Moody, Tetrahedron Lett. 2000, 41, 6897–6900;
- 10cM. C. Bagley, C. J. Moody, A. G. Pepper, Tetrahedron Lett. 2000, 41, 6901–6904;
- 10dC. J. Moody, K. J. Doyle, M. C. Elliott, T. J. Mowlem, J. Chem. Soc. Perkin Trans. 1 1997, 2413–2419;
- 10eC. J. Moody, K. J. Doyle, M. C. Elliott, T. J. Mowlem, Pure Appl. Chem. 1994, 66, 2107–2110.
- 11K. S. Feldman, K. J. Eastman, G. Lessene, Org. Lett. 2002, 4, 3524–3528.
- 12aH. C. Hang, E. Drotleff, G. I. Elliott, T. A. Ritsema, J. P. Konopelski, Synthesis 1999, 398–400;
- 12bJ. P. Konopelski, J. M. Hottenroth, H. M. Oltra, E. A. Veliz, Z. C. Yang, Synlett 1996, 609–611.
- 13A. Boto, M. Ling, G. Meek, G. Pattenden, Tetrahedron Lett. 1998, 39, 8167–8170.
- 14K. C. Nicolaou, M. Bella, D. Y.-K. Chen, X. Huang, T. Ling, S. A. Snyder, Angew. Chem. 2002, 114, 3645–3649;
10.1002/1521-3757(20020916)114:18<3645::AID-ANGE3645>3.0.CO;2-1 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3495–3499.10.1002/1521-3773(20020916)41:18<3495::AID-ANIE3495>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 15
- 15aK. C. Nicolaou, S. A. Snyder, K. B. Simonsen, A. E. Koumbis, Angew. Chem. 2000, 112, 3615–3620;
10.1002/1521-3757(20001002)112:19<3615::AID-ANGE3615>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3473–3478;10.1002/1521-3773(20001002)39:19<3473::AID-ANIE3473>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 15bK. C. Nicolaou, X. Huang, N. Giuseppone, P. Bheema Rao, M. Bella, M. V. Reddy, S. A. Snyder, Angew. Chem. 2001, 113, 4841–4845;
10.1002/1521-3757(20011217)113:24<4841::AID-ANGE4841>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4705–4709.10.1002/1521-3773(20011217)40:24<4705::AID-ANIE4705>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 16T. Ishiyama, M. Murata, N. Miyaura, J. Org. Chem. 1995, 60, 7508–7510.
- 17This step was inspired by related work: D. A. Klumpp, K. Y. Yeung, G. K. Surya Prakash, G. A. Olah, J. Org. Chem. 1998, 63, 4481–4484, and references therein.
- 18A. Padwa, D. Dehm, T. Oine, G. A. Lee, J. Am. Chem. Soc. 1975, 97, 1837–1845.
- 19The presence of the C11 carbonyl group retarded Suzuki coupling with 6 and led to the loss of the hydroxymethyl function on several intermediates as a result of the same type of fragmentation discussed in reference [15 a] that afforded benzofuran products; for a productive use of this pathway, see: K. C. Nicolaou, S. A. Snyder, A. Bigot, J. A. Pfefferkorn, Angew. Chem. 2000, 112, 1135–1138;
10.1002/(SICI)1521-3757(20000317)112:6<1135::AID-ANGE1135>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1093–1096.10.1002/(SICI)1521-3773(20000317)39:6<1093::AID-ANIE1093>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 20M. Nishiura, K. Katagiri, T. Imamoto, Bull. Chem. Soc. Jpn. 2001, 74, 1417–1424.
- 21Examination of other activating ligands for SmI2, such as our previously reported choice of HMPA (see reference [15 b]), did lead to the desired product, albeit in a lower yield.
- 22To the best of our knowledge, this particular reagent combination for oxazole synthesis from ketoamides has not been reported previously except by us (see reference [15 b]); however, there are examples with neat POCl3: R. L. Dow, J. Org. Chem. 1990, 55, 386–388.
- 23For examples of other conditions attempted to engender a Gabriel–Robinson dehydration, see:
- 23aP. Wipf, C. P. Miller, J. Org. Chem. 1993, 58, 3604–3606;
- 23bC. T. Brain, J. M. Paul, Synlett 1999, 1642–1644.
- 24For numerous examples of challenging macrolactamizations, see: D. L. Boger, S. H. Kim, Y. Mori, J.-H. Weng, O. Rogel, S. L. Castle, J. J. McAtee, J. Am. Chem. Soc. 2001, 123, 1862–1871.
- 25For another example of this set of conditions to remove an Fmoc group in the final step of a total synthesis (calicheamicin γ1I), see: K. C. Nicolaou, C. W. Hummel, M. Nakada, K. Shibayama, E. N. Pitsinos, H. Saimoto, Y. Mizuno, K.-U. Baldenius, A. L. Smith, J. Am. Chem. Soc. 1993, 115, 7625–7635.
- 26Although molecular models suggested the proximity of the reactive units, the steric hindrance within the system, in combination with the entropic penalties associated with the formation of a 12-membered ring with multiple elements of unsaturation from a far more flexible precursor, were the likely culprits that led to such recalcitrance.
- 27For other syntheses that benefited from this reagent combination, see:
- 27aK. C. Nicolaou, A. E. Koumbis, M. Takayanagi, S. Natarajan, N. F. Jain, T. Bando, H. Li, R. Hughes, Chem. Eur. J. 1999, 5, 2622–2647;
10.1002/(SICI)1521-3765(19990903)5:9<2622::AID-CHEM2622>3.0.CO;2-T CAS Web of Science® Google Scholar
- 27bT. Hu, J. S. Panek, J. Org. Chem. 1999, 64, 3000–3001;
- 27cD. A. Evans, M. R. Wood, B. W. Trotter, T. I. Richardson, J. C. Barrow, J. L. Katz, Angew. Chem. 1998, 110, 2864–2868;
10.1002/(SICI)1521-3757(19981002)110:19<2864::AID-ANGE2864>3.0.CO;2-R Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2700–2704.10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P CAS Web of Science® Google Scholar
- 28This result, combined with the yields obtained for the macrocyclization steps in our first total synthesis, indicates that the presence of either macrocyclic unit does not favor the formation of the second, once again revealing the truly constrained and compact nature of diazonamide A.
- 29Oxidative cleavage of benzyl ethers has been reported, although none of the substrates possessed nitrogen atoms; for an example, see: J. D. Prugh, C. S. Rooney, A. A. Deana, H. G. Ramjit, Tetrahedron Lett. 1985, 26, 2947.
- 30Selected data for compound 22: Rf=0.35 (EtOAc/hexanes=5:1); [α]
 (CH3OH, c=0.69)=−277.25; IR (film):
(CH3OH, c=0.69)=−277.25; IR (film):  max=3260, 2919, 1713, 1660, 1602, 1496, 1443, 1401, 1302, 1261, 1214, 1155, 1055, 750 cm−1; 1H NMR (600 MHz, CD3CN): δ=10.38 (s, 1 H), 8.42 (s, 1 H), 7.54 (d, J=7.9 Hz, 1 H), 7.42–7.33 (m, 7 H), 7.27 (br m, 1 H), 7.22 (d, J=7.0 Hz, 1 H), 7.10 (d, J=7.4 Hz, 1 H), 6.89–6.87 (m, 2 H), 6.82 (t, J=7.9 Hz, 1 H), 6.77 (d, J=7.9 Hz, 1 H), 6.73 (s, 1 H), 6.15 (d, J=7.9 Hz, 1 H), 5.08 (AB quart, J=12.7 Hz,
max=3260, 2919, 1713, 1660, 1602, 1496, 1443, 1401, 1302, 1261, 1214, 1155, 1055, 750 cm−1; 1H NMR (600 MHz, CD3CN): δ=10.38 (s, 1 H), 8.42 (s, 1 H), 7.54 (d, J=7.9 Hz, 1 H), 7.42–7.33 (m, 7 H), 7.27 (br m, 1 H), 7.22 (d, J=7.0 Hz, 1 H), 7.10 (d, J=7.4 Hz, 1 H), 6.89–6.87 (m, 2 H), 6.82 (t, J=7.9 Hz, 1 H), 6.77 (d, J=7.9 Hz, 1 H), 6.73 (s, 1 H), 6.15 (d, J=7.9 Hz, 1 H), 5.08 (AB quart, J=12.7 Hz,  AB=17.1 Hz, 2 H), 4.55 (t, J=7.4 Hz, 1 H), 4.24 (ddd, J=11.4, 8.8, 3.1 Hz, 1 H), 3.23 (t, J=12.7 Hz, 1 H), 2.68 (d, J=11.4 Hz, 1 H), 2.00 (m, 1 H), 1.01 (d, J=6.1 Hz, 3 H), 0.91 ppm (d, J=6.1 Hz, 3 H); 13C NMR (150 MHz, CDCl3): δ=174.9, 173.8, 164.3, 156.3, 154.6, 152.9, 152.8, 143.2, 140.6, 138.0, 135.9, 134.5, 131.6, 130.6, 130.0, 129.4, 129.3, 128.8, 128.6, 128.2, 127.2, 126.9, 124.8, 124.5, 123.2, 122.7, 116.6, 112.4, 98.3, 67.0, 57.8, 57.0, 56.4, 37.8, 31.0, 19.6, 19.0 ppm; HRMS (MALDI): calcd for C43H32Cl2N6O7+: 815.1782 [M+H+], found: 815.1778.
AB=17.1 Hz, 2 H), 4.55 (t, J=7.4 Hz, 1 H), 4.24 (ddd, J=11.4, 8.8, 3.1 Hz, 1 H), 3.23 (t, J=12.7 Hz, 1 H), 2.68 (d, J=11.4 Hz, 1 H), 2.00 (m, 1 H), 1.01 (d, J=6.1 Hz, 3 H), 0.91 ppm (d, J=6.1 Hz, 3 H); 13C NMR (150 MHz, CDCl3): δ=174.9, 173.8, 164.3, 156.3, 154.6, 152.9, 152.8, 143.2, 140.6, 138.0, 135.9, 134.5, 131.6, 130.6, 130.0, 129.4, 129.3, 128.8, 128.6, 128.2, 127.2, 126.9, 124.8, 124.5, 123.2, 122.7, 116.6, 112.4, 98.3, 67.0, 57.8, 57.0, 56.4, 37.8, 31.0, 19.6, 19.0 ppm; HRMS (MALDI): calcd for C43H32Cl2N6O7+: 815.1782 [M+H+], found: 815.1778.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.