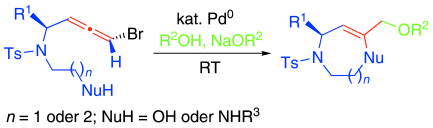Bromoallenes as Synthetic Equivalents of Allyl Dications: Synthesis of Medium-Sized Nitrogen Heterocycles through the Cyclization of Bromoallenes in the Presence of a Palladium(0) Catalyst and an Alcohol†
Hiroaki Ohno Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorHisao Hamaguchi
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorMiyo Ohata
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorTetsuaki Tanaka Prof. Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorHiroaki Ohno Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorHisao Hamaguchi
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorMiyo Ohata
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorTetsuaki Tanaka Prof. Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-8214
Search for more papers by this authorWe thank Prof. Dr. M. Mori, Hokkaido University, for useful information regarding the mechanism of the reaction. This work was supported by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports, and Culture of Japan. H.H. is grateful for a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science (JSPS).
Graphical Abstract
Eine hoch regio- und stereoselektive Cyclisierung zum Aufbau mittelgroßer Stickstoffheterocyclen wurde entwickelt (siehe Schema), die darauf beruht, dass Bromallene in Gegenwart eines Palladium(0)-Katalysators und eines Alkohols als Äquivalent für ein Allyldikation eingesetzt werden können. Der intramolekulare nucleophile Angriff erfolgt ausschließlich am mittleren Kohlenstoffatom der Allen-Einheit.
References
- 1For a recent review, see: P. A. Evans, A. B. Holmes, Tetrahedron 1991, 47, 9131–9166.
- 2For selected examples of seven-membered nitrogen heterocycles, see:
- 2aT. Duong, R. H. Prager, J. M. Tippett, A. D. Ward, D. I. Kerr, Aust. J. Chem. 1976, 29, 2667–2682;
- 2bC. Boros, S. M. Hamilton, B. Katz, P. Kulanthaivel, J. Antibiot. 1994, 47, 1010–1016;
- 2cY. Ishihara, K. Hirai, M. Miyamoto, G. Goto, J. Med. Chem. 1994, 37, 2292–2299;
- 2dF. Morís-Varas, X.-H. Qian, C.-H. Wong, J. Am. Chem. Soc. 1996, 118, 7647–7652;
- 2eG. L. Grunewald, V. H. Dahanukar, P. Ching, K. R. Criscione, J. Med. Chem. 1996, 39, 3539–3546.
- 3For selected examples of eight-membered nitrogen heterocycles, see:
- 3aB. Basil, E. C. J. Coffee, D. L. Gell, D. R. Maxwell, D. J. Sheffield, K. R. H. Wooldridge, J. Med. Chem. 1970, 13, 403–406;
- 3bD. L. Klayman, J. P. Scovill, J. F. Bartosevich, C. J. Mason, J. Med. Chem. 1979, 22, 1367–1373;
- 3cE. Vedejs, R. J. Galante, P. G. Goekjian, J. Am. Chem. Soc. 1998, 120, 3613–3622, and references therein.
- 4Most recent precedents for the synthesis of medium-sized nitrogen heterocycles are based on ring-closing metathesis. For a recent review, see:
- 4aM. E. Maier, Angew. Chem. 2000, 112, 2153–2157;
10.1002/1521-3757(20000616)112:12<2153::AID-ANGE2153>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2073–2077; for selected examples, see:10.1002/1521-3773(20000616)39:12<2073::AID-ANIE2073>3.0.CO;2-0 CAS PubMed Web of Science® Google Scholar
- 4bG. C. Fu, R. H. Grubbs, J. Am. Chem. Soc. 1992, 114, 7324–7325;
- 4cS. J. Miller, S.-H. Kim, Z.-R. Chen, R. H. Grubbs, J. Am. Chem. Soc. 1995, 117, 2108–2109;
- 4dM. S. Visser, N. M. Heron, M. T. Didiuk, J. F. Sagal, A. H. Hoveyda, J. Am. Chem. Soc. 1996, 118, 4291–4298;
- 4eL. A. Paquette, S. M. Leit, J. Am. Chem. Soc. 1999, 121, 8126–8127;
- 4fM. Mori, T. Kitamura, N. Sakakibara, Y. Sato, Org. Lett. 2000, 2, 543–545.
- 5For other recent syntheses of medium-sized nitrogen heterocycles, see:
- 5aX. Ouyang, A. S. Kiselyov, Tetrahedron 1999, 55, 8295–8302;
- 5bJ. Zhang, A. Jacobson, J. R. Rusche, W. Herlihy, J. Org. Chem. 1999, 64, 1074–1076;
- 5cR. Räcker, K. Döring, O. Reiser, J. Org. Chem. 2000, 65, 6932–6939;
- 5dA. I. Meyers, S. V. Downing, M. J. Weiser, J. Org. Chem. 2001, 66, 1413–1419;
- 5eT. J. Donohoe, A. Raoof, I. D. Linney, M. Helliwell, Org. Lett. 2001, 3, 861–864;
- 5fF. Iradier, R. G. Arrayás, J. C. Carretero, Org. Lett. 2001, 3, 2957–2960;
- 5gP. Tempest, V. Ma, M. G. Kelly, W. Jones, C. Hulme, Tetrahedron Lett. 2001, 42, 4963–4968; see also: ref. [3 c].
- 6For organocopper-mediated substitutions, see:
- 6aE. J. Corey, N. W. Boaz, Tetrahedron Lett. 1984, 25, 3059–3062;
- 6bA. M. Caporusso, C. Polizzi, L. Lardicci, Tetrahedron Lett. 1987, 28, 6073–6076;
- 6cF. D'Aniello, A. Mann, M. Taddei, J. Org. Chem. 1996, 61, 4870–4871;
- 6dF. Chemla, N. Bernard, J. Normant, Eur. J. Org. Chem. 1999, 2067–2078;
10.1002/(SICI)1099-0690(199909)1999:9<2067::AID-EJOC2067>3.0.CO;2-R CAS Web of Science® Google Scholar
- 6eA. M. Caporusso, S. Filippi, F. Barontini, P. Salvadori, Tetrahedron Lett. 2000, 41, 1227–1230;
- 6fJ. J. Conde, W. Mendelson, Tetrahedron Lett. 2000, 41, 811–814.
- 7For palladium-catalyzed cross-coupling reactions, see:
- 7aG. Märkl, P. Attenberger, J. Kellner, Tetrahedron Lett. 1988, 29, 3651–3654;
- 7bT. Gillmann, T. Hülsen, W. Massa, S. Wocadlo, Synlett 1995, 1257–1259;
- 7cR. W. Saalfrank, M. Haubner, C. Deutscher, W. Bauer, T. Clark, J. Org. Chem. 1999, 64, 6166–6168.
- 8For the formation of nucleophilic allenyl metal reagents, see: J. A. Marshall, N. D. Adams, J. Org. Chem. 1997, 62, 8976–8977.
- 9
- 9aH. Ohno, H. Hamaguchi, T. Tanaka, Org. Lett. 2001, 3, 2269–2271;
- 9bH. Ohno, K. Ando, H. Hamaguchi, Y. Takeoka, T. Tanaka, J. Am. Chem. Soc. 2002, 124, 15 255–15 266.
- 10It has been proposed that in the palladium-catalyzed reaction of propargyl carbonates, the protonation of a palladium carbene complex, generated by the reaction of an η1-allenyl palladium intermediate with an appropriate nucleophile, would produce the π-allyl palladium intermediate:
- 10aJ. Tsuji, H. Watanabe, I. Minami, I. Shimizu, J. Am. Chem. Soc. 1985, 107, 2196–2198; for an excellent review, see:
- 10bJ. Tsuji, T. Mandai, Angew. Chem. 1995, 107, 2830–2854;
10.1002/ange.19951072305 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2589–2612. More recently, an alternative mechanism was proposed, in which the protonation of a palladacyclobutene generates the same allyl palladium intermediate:
- 10cC. P. Casey, J. R. Nash, C. S. Yi, A. D. Selmeczy, S. Chung, D. R. Powell, R. K. Hayashi, J. Am. Chem. Soc. 1998, 120, 722–733. The reaction of a cationic η3-propargyl palladium species with a nucleophile to afford the allyl palladium complex has also been described:
- 10dK. Tsutsumi, S. Ogoshi, S. Nishiguchi, H. Kurosawa, J. Am. Chem. Soc. 1998, 120, 1938–1939;
- 10eK. Tsutsumi, T. Kawase, K. Kakiuchi, S. Ogoshi, Y. Okada, H. Kurosawa, Bull. Chem. Soc. Jpn. 1999, 72, 2687–2692.
- 11For recent examples, see:
- 11aN. Monteiro, A. Arnold, G. Balme, Synlett 1998, 1111–1113;
- 11bM. Yoshida, M. Ihara, Angew. Chem. 2001, 113, 636–639;
Angew. Chem. Int. Ed. 2001, 40, 616–619;
10.1002/1521-3773(20010202)40:3<616::AID-ANIE616>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 11cY. Kozawa, M. Mori, Tetrahedron Lett. 2001, 42, 4869–4873;
- 11dJ.-R. Labrosse, P. Lhoste, D. Sinou, J. Org. Chem. 2001, 66, 6634–6642;
- 11eY. Kozawa, M. Mori, Tetrahedron Lett. 2002, 43, 1499–1502.
- 12For other syntheses of medium-sized heterocycles from allenes, see:
- 12aR. W. Shaw, T. Gallagher, J. Chem. Soc. Perkin Trans. 1 1994, 3549–3555;
- 12bT. Okawara, S. Ehara, A. Takenaka, T. Hiwatashi, M. Furukawa, Heterocycles 1995, 41, 1709–1714;
- 12cR. Grigg, J. M. Sansano, Tetrahedron 1996, 52, 13 441–13 454;
- 12dB. M. Trost, P.-Y. Michellys, V. J. Gerusz, Angew. Chem. 1997, 109, 1837–1839;
10.1002/ange.19971091630 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1750–1753;
- 12eR. C. Larock, C. Tu, P. Pace, J. Org. Chem. 1998, 63, 6859–6866.
- 13
- 13aT. Ibuka, N. Mimura, H. Aoyama, M. Akaji, H. Ohno, Y. Miwa, T. Taga, K. Nakai, H. Tamamura, N. Fujii, Y. Yamamoto, J. Org. Chem. 1997, 62, 999;
- 13bT. Ibuka, N. Mimura, H. Ohno, K. Nakai, M. Akaji, H. Habashita, H. Tamamura, Y. Miwa, T. Taga, N. Fujii, Y. Yamamoto, J. Org. Chem. 1997, 62, 2982;
- 13cH. Ohno, K. Ishii, A. Honda, H. Tamamura, N. Fujii, Y. Takemoto, T. Ibuka, J. Chem. Soc. Perkin Trans. 1 1998, 3703;
- 13dH. Ohno, A. Toda, N. Fujii, Y. Miwa, T. Taga, Y. Yamaoka, E. Osawa, T. Ibuka, Tetrahedron Lett. 1999, 40, 1331;
- 13eN. Mimura, Y. Miwa, T. Ibuka, Y. Yamamoto, J. Org. Chem. 2002, 67, 5796–5801.
- 14The undesired methyl ether, produced by the SN2 substitution reaction of methoxide, was also detected in the product mixture.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.