Diastereoselective Temporary Silicon-Tethered Ring-Closing-Metathesis Reactions with Prochiral Alcohols: A New Approach to Long-Range Asymmetric Induction†
P. Andrew Evans Dr.
Department of Chemistry, Indiana University, Bloomington, IN 47405, USA, Fax: (+1) 812-855-8300
Search for more papers by this authorJian Cui Dr.
Department of Chemistry, Indiana University, Bloomington, IN 47405, USA, Fax: (+1) 812-855-8300
Search for more papers by this authorGerald P. Buffone
Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA
Search for more papers by this authorP. Andrew Evans Dr.
Department of Chemistry, Indiana University, Bloomington, IN 47405, USA, Fax: (+1) 812-855-8300
Search for more papers by this authorJian Cui Dr.
Department of Chemistry, Indiana University, Bloomington, IN 47405, USA, Fax: (+1) 812-855-8300
Search for more papers by this authorGerald P. Buffone
Department of Chemistry and Biochemistry, University of Delaware, Newark, DE 19716, USA
Search for more papers by this authorWe sincerely thank the National Institutes of Health (GM54623) for generous financial support. We also thank Johnson and Johnson for a Focused Giving Award, Pfizer Pharmaceuticals for the Creativity in Organic Chemistry Award, and Novartis Pharmaceuticals for an Academic Achievement Award. The Camille and Henry Dreyfus Foundation is thanked for a Camille Dreyfus Teacher-Scholar Award (P.A.E.).
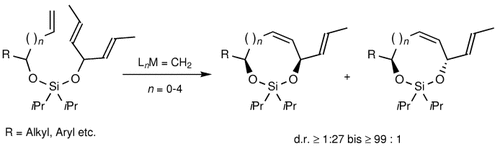
Graphical Abstract
Asymmetrische 1,4-, 1,5- und 1,6-Induktion: Diastereoselektive Ringschlussmetathese-Reaktionen, bei denen eine temporäre Siloxaneinheit genutzt wird, sind eine neue Strategie für weit reichende asymmetrische Induktion. Mit dieser Methode lassen sich cis-konfigurierte siebengliedrige Ringe des im Schema gezeigten Typs herstellen (n=0, d.r.=20:1); bei Verwendung längerer Alkenylgruppen (n=1–4) kehrt sich die Diastereoselektivität zugunsten des trans-Isomers um.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z50486_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on long-range asymmetric induction, see:
- 1aH. Sailes, A. Whiting, J. Chem. Soc. Perkin Trans. 1 2000, 1785–1805;
- 1bK. Mikami, M. Shimizu, H.-C. Zhang, B. E. Maryanoff, Tetrahedron 2001, 57, 2917–2951, and references therein.
- 2For recent examples of long-range asymmetric induction, see:
- 2aM. Date, Y. Tamai, T. Hattori, H. Takayama, Y. Kamikubo, S. Miyano, J. Chem. Soc. Perkin Trans. 1 2001, 645–653;
- 2bR. D. A. Hudson, C. E. Anson, M. F. Mahon, G. R. Stephenson, J. Organomet. Chem. 2001, 630, 88–103;
- 2cS. J. O'Malley, J. L. Leighton, Angew. Chem. 2001, 113, 2999–3001;
10.1002/1521-3757(20010803)113:15<2999::AID-ANGE2999>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2915–2917;10.1002/1521-3773(20010803)40:15<2915::AID-ANIE2915>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 2dM. W. Carson, G. Kim, S. J. Danishefsky, Angew. Chem. 2001, 113, 4585–4588;
10.1002/1521-3757(20011203)113:23<4585::AID-ANGE4585>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4453–4456;10.1002/1521-3773(20011203)40:23<4453::AID-ANIE4453>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 2eE. M. Flamme, W. R. Roush, J. Am. Chem. Soc. 2002, 124, 13 644–13 645, and references therein.
- 3For recent reviews on ring-closing metathesis, see:
- 3aR. H. Grubbs, S. Chang, Tetrahedron 1998, 54, 4413–4450;
- 3bU. K. Pandit, H. S. Overkleeft, B. C. Borer, H. Bieräugel, Eur. J. Org. Chem. 1999, 959–968;
10.1002/(SICI)1099-0690(199905)1999:5<959::AID-EJOC959>3.0.CO;2-K CAS Web of Science® Google Scholar
- 3cA. Fürstner, Angew. Chem. 2000, 112, 3140–3172;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012–3043, and references therein.10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 4G. C. Fu, R. H. Grubbs, J. Am. Chem. Soc. 1992, 114, 5426–5427.
- 5For a stereospecific approach to C2-symmetrical 1,4-diols, see:
- 5aP. A. Evans, V. S. Murthy, J. Org. Chem. 1998, 63, 6768–6769;
- 5bM. Lobbel, P. Köll, Tetrahedron: Asymmetry 2000, 11, 393–396.
- 6For examples of silicon-tethered ring-closing-metathesis cross-coupling reactions, see:
- 6aT. R. Hoye, M. A. Promo, Tetrahedron Lett. 1999, 40, 1429–1432;
- 6bJ.-G. Boiteau, P. Van de Weghe, J. Eustache, Tetrahedron Lett. 2001, 42, 239–242;
- 6cB. A. Harrison, G. L. Verdine, Org. Lett. 2001, 3, 2157–2159.
- 7For a related example of an enantioselective RCM using a prochiral alcohol, see: A. F. Kiely, J. A. Jernelius, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 2868–2869.
- 8For examples of diastereoselective ring-closing-metathesis reactions, see:
- 8aC. M. Huwe, J. Velder, S. Blechert, Angew. Chem. 1996, 108, 2542–2544;
10.1002/ange.19961082027 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2376–2378;
- 8bS. D. Burke, M. Müller, C. M. Beaudry, Org. Lett. 1999, 1, 1827–1829;
- 8cH. Oguri, S.-Y. Sasaki, T. Oishi, M. Hirama, Tetrahedron Lett. 1999, 40, 5405–5408;
- 8dM. Lautens, G. Hughes, Angew. Chem. 1999, 111, 160–162;
Angew. Chem. Int. Ed. 1999, 38, 129–131;
10.1002/(SICI)1521-3773(19990115)38:1/2<129::AID-ANIE129>3.0.CO;2-I CAS Web of Science® Google Scholar
- 8eG. C. Lloyd-Jones, M. Murray, R. A. Stentiford, P. A. Worthington, Eur. J. Org. Chem. 2000, 975–985;
10.1002/(SICI)1099-0690(200003)2000:6<975::AID-EJOC975>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 8fD. J. Wallace, C. J. Cowden, D. J. Kennedy, M. S. Ashwood, I. F. Cottrell, U.-H. Dolling, Tetrahedron Lett. 2000, 41, 2027–2029;
- 8gB. Schmidt, M. Westhus, Tetrahedron 2000, 56, 2421–2426;
- 8hD. S. Stoianova, P. R. Hanson, Org. Lett. 2000, 2, 1769–1772;
- 8iY. Fukuda, H. Sasaki, M. Shindo, K. Shishido, Tetrahedron Lett. 2002, 43, 2047–2049;
- 8jM. Ogasawara, T. Nagano, T. Hayashi, J. Am. Chem. Soc. 2002, 124, 9068–9069.
- 9For reviews on temporary silicon-tethered strategies, see:
- 9aL. Fensterbank, M. Malacria, S. M. Sieburth, Synthesis 1997, 813–854;
- 9bD. R. Gauthier, Jr.,K. S. Zandi, K. J. Shea, Tetrahedron 1998, 54, 2289–2338.
- 10S. M. Sieburth, L. Fensterbank, J. Org. Chem. 1992, 57, 5279–5281.
- 11
- 11aR. R. Schrock, J. S. Murdzek, G. C. Bazan, J. Robbins, M. DiMare, M. O'Regan, J. Am. Chem. Soc. 1990, 112, 3875–3886;
- 11bP. Schwab, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1996, 118, 100–110;
- 11cM. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953–956.
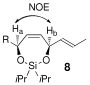
- 12The major stereoisomer was confirmed from nOe experiments in each case (8 a–i). Details are available in the Supporting Information.
- 13The silyl tether can be readily removed in each case to afford the corresponding diol. For example, treatment of 8 f with 5 % aqueous HF at room temperature furnished the bisallylic 1,4-diol 16 in 99 % yield.
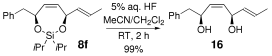
- 14Although Grubbs' catalyst 10 was not optimal for the larger rings, it also furnished 15 as the major diastereoisomer, which confirmed that the catalyst was not responsible for the reversal in selectivity.
- 15The diastereoselectivities in Figure 3, refer to crude product ratios, whereas the selectivities in Table 3 are for the purified silaketals 14 a–d and 15 a–d.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.