Clinical parameter-guided initial resuscitation in adult patients with septic shock: A systematic review and network meta-analysis
Tetsuya Yumoto and Tomoki Kuribara contributed equally to this work.
Abstract
Aim
To identify the most useful tissue perfusion parameter for initial resuscitation in sepsis/septic shock adults using a network meta-analysis.
Methods
We searched major databases until December 2022 for randomized trials comparing four tissue perfusion parameters or against usual care. The primary outcome was short-term mortality up to 90 days. The Confidence in Network Meta-Analysis web application was used to assess the quality of evidence.
Results
Seventeen trials were identified. Lactate-guided therapy (risk ratios, 0.59; 95% confidence intervals [0.45–0.76]; high certainty) and capillary refill time-guided therapy (risk ratios, 0.53; 95% confidence intervals [0.33–0.86]; high certainty) were significantly associated with lower short-term mortality compared with usual care, whereas central venous oxygen saturation-guided therapy (risk ratio, 1.50; 95% confidence intervals [1.16–1.94]; moderate certainty) increased the risk of short-term mortality compared with lactate-guided therapy.
Conclusions
Lactate or capillary refill time-guided initial resuscitation for sepsis/septic shock patients may decrease short-term mortality. More research is essential to personalize and optimize treatment strategies for septic shock resuscitation.
INTRODUCTION
Sepsis is a life-threatening condition marked by organ dysfunction from a dysregulated response to infection.1 Immediate treatment, particularly fluid resuscitation and vasopressors is critical in patients with possible impaired tissue perfusion or septic shock. Balancing fluid input is essential; excessive resuscitation can lead to complications such as pulmonary edema and increased risk of death.2 Regular assessment of patients allows clinicians to adjust treatment, but there is uncertainty about which variables best optimize organ perfusion.
Currently, blood lactate assessment is standard for suspected sepsis because it is central to its definition.1 Although elevated lactate levels are often associated with tissue hypoperfusion, they can also be influenced by other factors such as aerobic glycolysis or mitochondrial dysfunction, making them an imperfect and occasionally misleading indicator.3 This uncertainty underscores the weak recommendation and low-quality evidence for lactate-guided therapy.2 A trial of early goal-directed therapy (EGDT), specifically focused on central venous oxygen saturation (ScvO2), showed no improvement over standard care.4 Meanwhile, the veno-arterial difference in the partial pressure of carbon dioxide, also known as the PCO2 gap, has been proposed as a potentially more dependable alternative to ScvO2 or blood lactate in indicating tissue hypoperfusion.5 A recent trial highlighted better outcomes using capillary refill time (CRT) over lactate monitoring, with the CRT group showing reduced Sequential Organ Failure Assessment (SOFA) scores and a trend of lower mortality at 28 days.6
Given the fragmented evidence and limited data on the best monitoring strategies and organ perfusion variables in sepsis, we undertook a network meta-analysis (NMA). Our goal was to determine which tissue perfusion or clinical parameter-guided therapy is most useful in improving outcomes for adults with sepsis or septic shock.
MATERIALS AND METHODS
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension statement for reporting NMAs (PRISMA-NMA) (Table S1S1). The protocol was registered on protocols.io (74175).
Eligibility criteria
We included randomized controlled trials (RCTs) analyzing the effectiveness of various tissue perfusion parameters, or comparing them to standard care, for initial resuscitation in adult sepsis/septic shock patients. Parameters studied were lactate, CRT, ScvO2/mixed venous oxygen saturation (SvO2), and the ratio of veno-arterial carbon dioxide tension difference to arterial–venous oxygen content difference P(v–a)CO2/C(a–v)O2. SvO2-guided therapy was considered equivalent to ScvO2.7 EGDT trials were categorized under ScvO2 because of its key role in septic shock resuscitation.8 Usual care was resuscitation without specific tissue perfusion targets. In multi-arm trials, any of the two arms listed above, including usual care, were compared to reflect each comparison. Multi-arm trials assessing different goal settings of a single parameter were combined. Studies on mixed populations of critically ill patients involving the subgroup of patients with severe sepsis or septic shock were also included. The primary outcome was short-term mortality (up to 90 days) or in-hospital mortality if time-specific data was not provided. Secondary outcomes included intensive care unit (ICU) mortality, ventilator-free days at 28 days, and ICU length of stay.
Information sources and search
We searched the Cochrane Central Register of Controlled Trials, MEDLINE via PubMed, Web of Science, and the ICHUSHI database (a national database of Japanese research papers) until December 2022. Our detailed search strategy is available in Table S2. We also searched the World Health Organization International Clinical Trials platforms Search Portal and ClinicalTrials.gov. for ongoing trials up to December 2022.
Study selection and data collection process
Two of the five authors screened titles and abstracts, with full-text reviews for final inclusion. Disagreements were settled by a third researcher. Similarly, two reviewers independently extracted the data. Study authors were contacted to resolve any queries.
Risk of bias within individual studies
Two of the five reviewers assessed the risk of bias independently based on the Cochrane Risk of Bias tool version 2 (RoB 2). Any disagreement was handled by a third reviewer. We rated each risk of bias as “low risk,” “some concerns,” or “high risk” of bias.
Analyses
We conducted a pairwise meta-analysis for every direct comparison using RevMan 5.4. For categorical outcomes, the effect sizes were expressed as risk ratios (RR) with their 95% confidence intervals (CI), whereas weighted mean differences (MD) with 95% CI were used for continuous outcomes. We used random-effects models to estimate the pooled effect sizes. The NMA was conducted using Stata version 17 statistical software (Stata-Corp LP, College Station, TX, USA). We created network plots showing direct comparisons between tissue perfusion parameters. Pooled RRs or weighted MDs with their 95% CIs, as appropriate, were estimated using a multivariate random-effects meta-analysis. We also calculated the surface under the cumulative ranking curve (SUCRA) to estimate ranking probabilities of each parameter. Certainty of evidence for each outcome was evaluated using the Confidence in Network Meta-Analysis (CINeMA) approach.9 To ensure our results' robustness, we conducted sensitivity analyses for the primary outcome by 1) excluding trials with high risk of bias, 2) excluding pre-2004 trials following the first Survival Sepsis Campaign, and 3) excluding trials in mixed critically ill patients with sepsis or septic shock.
RESULTS
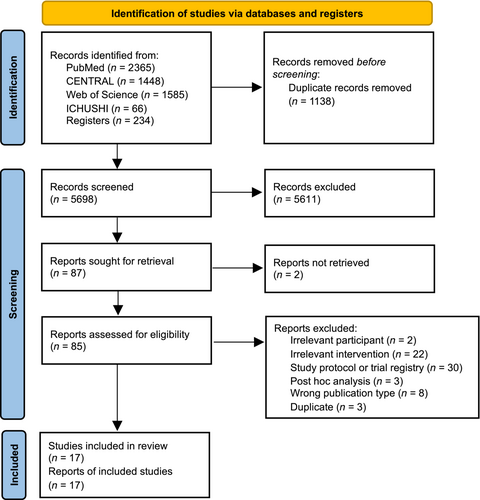
Figure 1 shows the PRISMA flow diagram for study selection. Seventeen studies were finalized for analysis.
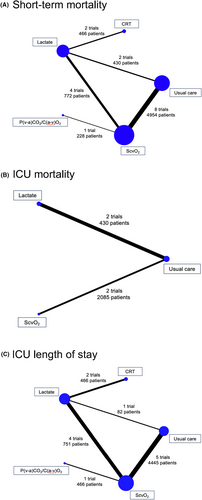
Network geometry
Figure 2 illustrates the network plot for primary and secondary outcomes. Eight trials compared ScvO2-guided therapy with usual care,10-17 four trials compared lactate-guided therapy with ScvO2-guided therapy,18-21 two trials compared lactate-guided therapy with usual care,22, 23 two trials compared CRT-guided therapy with lactate-guided therapy,6, 24 and one trial compared P(v–a)CO2/C(a–v)O2-guided therapy with ScvO2-guided therapy.25
Study characteristics
Table 1 demonstrates the main characteristics of the selected studies. Three trials included mixed critically ill patients with sepsis or septic shock.12, 17, 22 Of the remaining 14 trials of patients with sepsis/septic shock, two targeted patients with pneumonia and those 60 years old or older.18, 23
| Source | Funding | Country | Total no. of patients | Participants | Exposure/control age, mean (SD), year | Intervention | Comparison | Treatment period | Mortality assessed | Secondary outcomes assessed |
|---|---|---|---|---|---|---|---|---|---|---|
| Gattinoni et al.17 | Eli Lilly Italy and Abbott Italy | Italy | 762a | Critically ill (mixed) | 62.4 (15.4)/61.3 (16.2) | SvO2 (≥70%) | Usual | 5 days | ICU |
ICU mortality ICU length of stay |
| Rivers et al.10 | Henry Ford Health Systems Fund for Research, a Weatherby Healthcare Resuscitation Fellowship, Edwards Lifesciences, and Nova Biomedical | US | 263 | Septic shock | 67.1 (17.4)/64.4 (17.1) | EGDT (ScvO2 ≥70%) | Usual | 6 h | In-hospital | N/A |
| Wang et al.11 | Undisclosed | China | 33 | Septic shock | 33 (13)/36 (14) | EGDT (ScvO2 ≥70%) | Usual | 6 to 10 h | 14-day | N/A |
| Chen et al.12 | Undisclosed | China | 273 | Critically ill (MODS) | 51.27 (16.76)/53.71 (16.62) | EGDT (ScvO2 ≥70%) | Usual | 6 h | ICU | ICU mortality |
| Jansen et al.22 | Undisclosed | Netherlands | 348 | Critically ill (lactate level >3.0 mEq/L) | 61 (15)/62 (18) | Lactate (decrease >10% every 2 h) | Usual | 8 h | ICU | ICU mortality |
| Early Goal-Directed Therapy Collaborative Group of Zhejiang Province13 | Zhejiang Provincial Medical and Health Key Scientific Research Project, Zhejiang Province Natural Science Foundation of China, and Zhejiang Provincial Health High-level Innovative Talent Fund Project | China | 303 | Severe sepsis or septic shock | 68.9 (15.6)/67.7 (18.1) | EGDT (ScvO2 ≥70%) | Usual | 6 h | 28-day |
Ventilated days ICU length of stay |
| Jones et al.19 | National Institutes of Health | US | 300 | Septic shock | 59.8 (17.6)/61.6 (17.6) | Lactate (decrease >10% every 2 h) | ScvO2 (≥70%) | 6 h | In-hospital |
Ventilated days Ventilator-free days ICU length of stay |
| Tian et al.18 | Shandong Natural Science Funding | China | 62b | Pneumonia with septic shock | 51.86 (19.38)/46.18 (16.28) | Lactate (decrease >10% or 30% every 2 h) | ScvO2 (>70%) | 6 h | 28-day | ICU length of stay |
| Yu et al.20 | Hebei Province Medical Scientific Research Key Project | China | 50 | Septic shock | 61 (12)/59 (18) | Lactate (decrease >10% every 3 h) | ScvO2 (≥70%) | 6 h | 28-day | ICU length of stay |
| ARISE Investigators14 | National Health and Medical Research Council of Australia and the Alfred Foundation | Australia and New Zealand, et al. | 1588 | Septic shock | 62.7 (16.4)/63.1 (16.5) | EGDT (ScvO2 ≥70%) | Usual | 6 h | 90-day |
ICU mortality ICU length of stay |
| ProCESS Investigators15 | National Institute of General Medical Sciences | US | 1351c | Septic shock | 60 (16.4)/62 (16.0) | EGDT (ScvO2 ≥70%) | Usual | 6 h | 90-day |
Ventilated days ICU length of stay |
| Mouncey et al.16 | United Kingdom National Institute for Health Research Health Technology Assessment Programme | UK | 1260 | Septic shock | 66.4 (14.6)/64.3 (15.5) | EGDT (ScvO2 ≥70%) | Usual | 6 h | 90-day |
Ventilator-free days ICU length of stay |
| Zhou et al.21 | Health Scientific Research in the Public Interest Program | China | 360 | Septic shock | 56 (44, 66)/56 (40, 67) | Lactate (decrease >10% every 2 h) | ScvO2 (≥70%) | 6 h | 60-day |
Ventilated days ICU length of stay |
| Su et al.25 | N/A | China | 228 | Severe sepsis or septic shock | 63 (17)/62 (17) | P(v–a)CO2/C(a–v)O2 ≥ 1.8 | ScvO2 (≥70%) | 3 days | 60-day |
Ventilator-free days ICU length of stay |
| Hernández et al.6 | Pontificia Universidad Católica of Chile | Argentina, Chile, Colombia, Ecuador, Uruguay | 424 | Septic shock | 62 (17)/64 (17) | CRT (≤3 s) | Lactate (decrease >20% every 2 h) | 8 h | 90-day |
Ventilator-free days ICU length of stay |
| Castro et al.24 | FONDECYT Chile Grant project | Chile | 42 | Septic shock | 51 (45, 75)/66 (55, 75) | CRT (≤3 s) | Lactate (decrease >20% every 2 h) | 6 h | 28-day | ICU length of stay |
| Chen et al.23 | N/A | China | 82 | Septic shock (age ≥60) | 72.4 (9.2)/70.9 (8.3) | Lactate (decrease >20% every 2 h) | Usual | 6 h | ICU |
ICU mortality Ventilated days ICU length of stay |
- Abbreviations: CRT, capillary refill time; EGDT, early goal-directed therapy; ICU, intensive care unit; MODS, multiple organ dysfunction syndrome; ScvO2, central venous oxygen saturation; SvO2, mixed venous oxygen saturation; P(v–a)CO2/C(a–v)O2, ratio of veno-arterial carbon dioxide tension difference to arterial–venous oxygen content difference; SD, standard deviation; UK, United Kingdom; US, United States.
- a Three-arm trials, including cardiac index group, which was out of our scope.
- b Three-arm trials, including lactate clearance of 10%, 30%, and usual care.
- c Three-arm trials comparing EGDT to protocol-based standard therapy, and usual care.
Short-term mortality up to 90 days
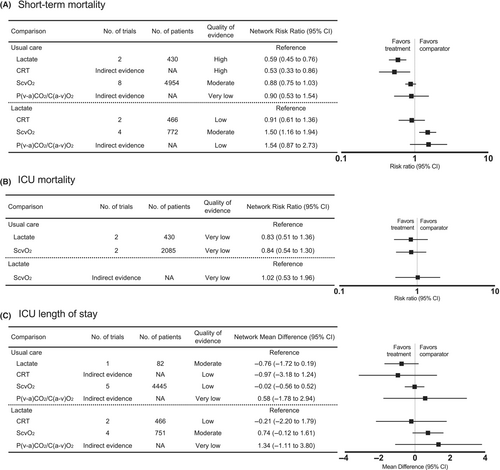
Seventeen trials were included in the short-term mortality analysis. The results of pairwise meta-analysis are provided in Figure S1 and Table S3). Lactate-guided (RR, 0.59; 95% CI [0.45–0.76]; high certainty) and CRT-guided therapies (RR, 0.53; 95% CI [0.33–0.86]; high certainty) significantly reduced short-term mortality compared to usual care. In contrast, ScvO2-guided (RR, 0.88; 95% CI [0.75–1.03]; moderate certainty) and P(v–a)CO2/C(a–v)O2-guided therapies (RR, 0.90; 95% CI [0.53–1.54]; very low certainty) did not. Considering lactate-guided therapy as the reference, neither CRT-guided therapy (RR, 0.91; 95% CI [0.61–1.36]; low certainty) nor P(v–a)CO2/C(a–v)O2-guided therapy (RR, 1.54; 95% CI [0.87–2.73]; low certainty) lowered short-term mortality, whereas ScvO2-guided therapy (RR, 1.50; 95% CI [1.16–1.94]; moderate certainty) increased the risk of short-term mortality (Figure 3 and Table S4). The SUCRA statistic is shown in Table 2.
| Rank | Usual care | CRT | Lactate | P(v–a)CO2/C(a–v)O2 | ScvO2 |
|---|---|---|---|---|---|
| Short-term mortality | |||||
| Best | 0.0 | 66.3 | 29.8 | 3.9 | 0.0 |
| 2nd | 0.1 | 27.2 | 65.0 | 6.4 | 1.3 |
| 3rd | 3.0 | 4.9 | 5.1 | 35.0 | 52.0 |
| 4th | 33.7 | 1.3 | 0.1 | 20.3 | 44.7 |
| Worst | 63.2 | 0.3 | 0.0 | 34.5 | 2.0 |
| Mean rank | 4.6 | 1.4 | 1.8 | 3.8 | 3.5 |
| SUCRA | 0.1 | 0.9 | 0.8 | 0.3 | 0.4 |
| ICU mortality | |||||
| Best | 0.0 | 6.7 | 93.3 | ||
| 2nd | 10.1 | 83.5 | 6.4 | ||
| Worst | 89.9 | 9.8 | 0.3 | ||
| Mean rank | 2.9 | 2.0 | 1.1 | ||
| SUCRA | 0.1 | 0.5 | 1.0 | ||
| ICU length of stay | |||||
| Best | 1.8 | 53.7 | 35.2 | 8.3 | 1.0 |
| 2nd | 9.9 | 20.7 | 51.9 | 8.6 | 9.0 |
| 3rd | 32.9 | 6.9 | 10.4 | 11.7 | 38.1 |
| 4th | 39.1 | 9.8 | 2.0 | 8.2 | 40.9 |
| Worst | 16.4 | 8.8 | 0.6 | 63.3 | 11.0 |
| Mean rank | 3.6 | 2.0 | 1.8 | 4.1 | 3.5 |
| SUCRA | 0.4 | 0.8 | 0.8 | 0.2 | 0.4 |
- Abbreviations: CRT, capillary refill time; ICU, intensive care unit; ScvO2, central venous oxygen saturation; SUCRA, surface under the cumulative ranking; P(v–a)CO2/C(a–v)O2, ratio of veno–arterial carbon dioxide tension difference to arterial–venous oxygen content difference.
Secondary outcomes
ICU mortality was evaluated from five studies. Pairwise comparisons of the individual studies are presented in Figure S2 and Table S3. Compared to usual care as the reference, neither lactate-guided therapy nor ScvO2-guided therapy was associated with decreased ICU mortality. Lactate-guided therapy was not superior to ScvO2-guided therapy (Figure 3 and Table S4). SUCRA ranking is shown in Table 2.
Thirteen trials reported on ICU length of stay. Pairwise comparisons are provided in Figure S3 and Table S3). CRT-, lactate-, ScvO2-, and P(v–a)CO2/C(a–v)O2-guided therapy were not associated with shorter ICU length of stay compared with usual care (Figure 3 and Table S4). Ranking probabilities is shown in Table 2.
Four trials reporting ventilator-free days were identified. Pairwise comparisons are shown in Figure S4 and Table S3. The limited number of trials providing information on ventilator-free days did not allow us to perform NMA.
Sensitivity analyses
After excluding trials with a high risk of bias, those before 2004, and those on mixed critically ill patients, lactate and CRT-guided therapies still showed lower short-term mortality than usual care, whereas ScvO2-guided therapy had higher mortality risk than lactate-guided therapy (Table S5 and Figure S5).
DISCUSSION
Based on an NMA of 17 studies with 6850 participants across five interventions, lactate or CRT-guided resuscitation showed significantly lower mortality up to 90 days in adults with sepsis or septic shock compared to usual care, whereas ScvO2-guided therapy had a higher mortality risk than lactate-guided therapy.
Our results align with recent meta-analyses, highlighting lactate-guided therapy's superiority in reducing ICU mortality over ScvO2-guided therapy.26 Past research indicated sepsis affects tissue oxygen balance, as evidenced by decreased ScvO2 or SvO2.8 In 2001, the Rivers trial showed EGDT, primarily based on continuous ScvO2 monitoring, improved ICU mortality compared to standard care.10 However, three subsequent trials disagreed.14-16 This inconsistency stems from differing initial ScvO2 values across studies: 49% in the Rivers trial versus around 71% in the latter three. Therefore, many patients in the later trials might have already reached desired ScvO2 levels at the start. Given ScvO2's role in indicating tissue oxygenation and its link to mortality, those with “normal” ScvO2 might not have received aggressive resuscitation.27
The 2004 Survival Sepsis Campaign promoted standardized care emphasizing large volume fluid resuscitation, although concerns about fluid overload emerged.28 The ANDROMEDA-SHOCK trial compared CRT, a simple peripheral perfusion parameter, with lactate-guided therapy for septic shock. Despite similar 28-day mortality rates, CRT patients showed better 72-hour SOFA scores and received less initial fluid.6 Post-hoc analyses revealed higher mortality in patients normalized by CRT, but further treated with lactate-guided fluids, suggesting curtailing aggressive resuscitation once CRT is normal.29 Our NMA found no difference between the two therapies, but CRT had superior short-term mortality outcomes. P(v–a)CO2/C(a–v)O2-guided therapy showed no clear advantage in short-term mortality over usual care or lactate-guided therapy with very low and low certainty of evidence, respectively. The results may be difficult to interpret because of indirect evidence based on only a single study comparing P(v–a)CO2/C(a–v)O2-guided therapy and ScvO2-guided therapy.25
Recent trials have tested individualized fluid resuscitation strategies, often using lactate levels or knee mottling as benchmarks.30 Lactate-guided resuscitation is a mainstay for septic shock, aligning with our NMA findings. Given sepsis's complexity, combining lactate with parameters such as CRT may be effective. Continued research is crucial to refine septic shock resuscitation guidelines.
This study has several limitations. First, the validity of NMA relies on the assumption of similar study populations, but five of 17 trials had diverse patient groups. Second, with evolving sepsis management guidelines, there is clinical variance across studies. Third, although our findings withstood sensitivity analyses, the few direct intervention comparisons led to sparse networks. Fourth, targets for lactate clearance varied between studies, and minimal data on ventilator-free days prevented an NMA on that outcome. Last, although long-term mortality was not examined because of an extreme paucity of studies addressing this specific outcome, our analysis of ICU mortality was similarly constrained by limited networks and few studies.
CONCLUSIONS
In this NMA, lactate or CRT guidance for septic shock resuscitation appears to reduce short-term mortality. However, because of significant heterogeneity and the need for individualized treatments, results should be interpreted cautiously. Further research is essential not only to examine ICU and long-term mortality, but also to explore other clinical outcomes and optimal treatment combinations for initial resuscitation, thereby creating a comprehensive evidence base for septic shock resuscitation strategies.
ACKNOWLEDGEMENTS
We thank Christine Burr for English editing of the article. We also like to thank the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2024 (J-SSCG 2024) committee for supporting this project.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the Research Protocol: N/A.
Informed Consent: N/A.
Registry and the Registration No. of The Study/Trial: The study protocol was registered on protocols.io (74175).
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




