Segmental arterial mediolysis with a ruptured visceral artery on two consecutive days
Abstract
Background
We describe a case of segmental arterial mediolysis in which a vessel ruptured on two consecutive days.
Case Presentation
A 69-year-old man presented with sudden-onset abdominal pain. Computed tomography showed a hematoma in the gastric wall. The patient was discharged after the pain was relieved but returned 8 h later with abdominal pain and shock. Repeated computed tomography revealed a massive intra-abdominal hemorrhage without previous aneurysm formation. Emergency angiography and coil embolization were successfully carried out. Segmental arterial mediolysis was diagnosed after irregular vasodilated lesions were observed in multiple arteries.
Conclusion
This case suggests that accurately predicting the next vessel rupture is difficult. For patients experiencing intra-abdominal bleeding with segmental arterial mediolysis, we suggest treating only ruptured aneurysms and closely following-up unruptured aneurysms.
BACKGROUND
Segmental arterial mediolysis (SAM), a noninflammatory and nonatherosclerotic degenerative disease of the abdominal visceral arteries (AVAs),1 is known to cause aneurysms in AVAs. Ruptured AVAs result in intra-abdominal bleeding and require emergency treatment; however, unruptured aneurysms often resolve spontaneously.2-4
In this report, we describe a case of SAM in which a vessel ruptured on two consecutive days without aneurysm formation.
CASE PRESENTATION
A 69-year-old man was brought to our emergency department with a chief complaint of sudden-onset abdominal pain. He was alert, and his vital signs were as follows: respiratory rate, 20 breaths/min; heart rate, 63 b.p.m.; blood pressure, 213/117 mmHg; and saturation of percutaneous oxygen, 98%. Abdominal tenderness and signs of peritoneal irritation were observed. Hematological findings are presented in Table 1. Noncontrast-enhanced abdominal computed tomography (CT) revealed a swelling of the anterior gastric wall (Figure 1A). No diagnosis was made at this time, although it was later diagnosed as gastric wall hematoma. Acetaminophen administration relieved his pain, and he was discharged.
| Day 1 (ED) | Day 2 (ED) | Day 3 (Ward) | |
|---|---|---|---|
| White blood cells (103/μL) | 10.2 | 15.6 | 10.2 |
| Hemoglobin (g/dL) | 12.5 | 9.6 | 6.8 |
| Platelets (109/L) | 50 | 42 | 66 |
| Prothrombin time (INR) | 1.04 | 1.13 | |
| APTT (second) | 28.1 | 27.6 | |
| CRP (mg/dL) | 0.07 | 0.13 | 3.91 |
| Antinuclear antibody (times) | <40 | ||
| PR-3-ANCA (U/mL) | <1.0 | ||
| MPO-ANCA (U/mL) | <1.0 |
- Abbreviations: APTT, activated partial thromboplastin time; CRP, C-reactive protein; ED, emergency department; INR, international normalized ratio; MPO-ANCA, myeloperoxidase antineutrophil cytoplasmic antibody; PR-3-ANCA, proteinase-3-antineutrophil cytoplasmic antibody.
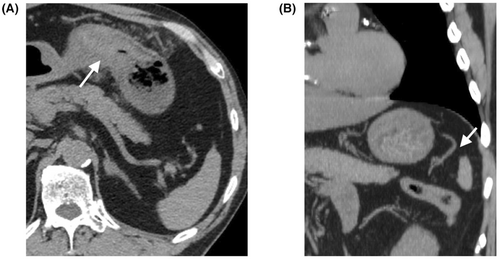
Approximately 8 h after discharge, the patient returned to the emergency department with abdominal pain and shock. He was pale and cold; his vital signs were as follows: Glasgow Coma Scale, 14 (E3V5M6); respiratory rate, 36 breaths/min; heart rate, 80 b.p.m.; blood pressure, 51/32 mmHg; and saturation of percutaneous oxygen, 100%. Abdominal tenderness and signs of peritoneal irritation were observed on abdominal examination. Hematological findings are presented in Table 1. Contrast-enhanced CT revealed a massive intra-abdominal hemorrhage and pseudoaneurysm in the gastrosplenic mesentery (Figure 2A,B). No aneurysm in the short gastric artery (SGA) was detected on the initial CT image (Figure 1B). Vascular irregularities were also noted in the left gastric artery (LGA) and the left and right gastroepiploic arteries (GEAs) on second CT, but with no evidence of contrast extravasation; hence, they were not considered as a source of bleeding.
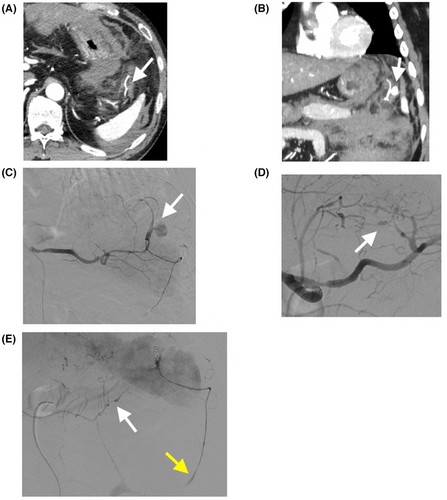
The patient was intubated because of persistent hypotension. Emergency angiography (AG) was carried out. Angiography showed a slightly dilated lesion with contrast extravasation of the SGA (Figure 2C), and coil embolization was successfully performed (Figure 2E). After hemostasis, AG also showed irregular vasodilated lesions in the LGA and left and right GEAs (Figure 2D,E). There were no inflammatory findings on hematology. Considering all of these findings, we diagnosed SAM. His condition stabilized after hemostasis and blood transfusion. He was extubated 3 days post-AG and discharged from the intensive care unit on foot 10 days post-AG. One-month follow-up CT showed regression of the vascular lesions.
DISCUSSION
In our case, SAM was diagnosed by clinical, radiologic, and laboratory findings. The radiologic feature of SAM is defined as a string-of-beads appearance or the presence of multiple fusiform aneurysms.3, 4 Angiography of the patient showed irregular vasodilated lesions in the LGA and left and right GEAs, which were interpretable as string-of-beads appearance or multiple fusiform aneurysms. Additionally, it showed slight abnormalities on SGA and pancreatic duodenal arteries, and no abnormalities on hepatic and splenic arteries. Computed tomography scan showed no abnormalities on supra or infra mesenteric arteries or renal arteries. The differential diagnosis of SAM includes atherosclerosis, fibromuscular dysplasia, infection, connective tissue diseases (e.g., Behçet's disease and polyarteritis nodosa), and inherited defects in vessel wall structural proteins (e.g., type IV Ehlers–Danlos syndrome and Marfan syndrome).5
In our case, the renal arteries were not involved, and age/sex characteristics were different from those of fibromuscular dysplasia. The patient did not have the physical features of Ehlers–Danlos syndrome or Marfan syndrome and was older in age of onset. There were no inflammatory findings or detection of negative markers of autoimmune disease, which suggested no systemic infections or inflammatory vasculitis. Atherosclerosis typically occurs at the branch points of vessels5; although this patient had calcification of arteries, it was unlikely that abnormal findings were due to atherosclerosis.
For many years, surgery was the first-choice treatment for SAM.2 However, in 2014, Pillai et al. stated that the choice of treatment for SAM should be based on clinical findings, the location of the diseased vessels, and the presence or absence of organ ischemia.6 Due to improvements in technology and expertise, intervention radiology (IR) for ruptured aneurysms has recently become the preferred management method for patients with SAM and intra-abdominal hemorrhage.2-4, 6 Surgery is selected for cases with severe shock or organ ischemia or when the rupture cannot be controlled by IR.
In the case of SAM, unruptured aneurysms did not rupture during the subsequent course and either disappeared or were maintained during follow-up.3 This study noted that the current general treatment policy is to undertake arterial embolization or surgical procedures only for ruptured aneurysms and carefully monitor other aneurysms.3 Reportedly, results of the natural history of 70 unruptured aneurysms due to SAM showed no change in the natural history in 64%, size reduction in 13%, and disappearance in 19% of the aneurysms. Unruptured aneurysms due to SAM at initial SAM diagnosis are likely to be stable or resolved; thus, conservative management seems appropriate.4
Segmental arterial mediolysis begins with mediolysis, which involves the degeneration and lysis of the outer smooth muscle cells in the media. This is followed by a tear that separates the outer medial muscle, resulting in a patchy loss of the external elastic lamina. Eventually, the internal elastic lamina and intima are destroyed, leading to the formation of arterial gaps. These gaps can cause intramural hematomas and dissecting aneurysms when blood flows through the media tear.6
In the present case, initial gastric hematoma was the dominant lesion of LGA, and the site of initial rupture. This was followed by the rupture of the SGA, several hours later, resulting in intra-abdominal hemorrhage. We believe that mediolysis caused an arterial gap several hours before the rupture of the SGA, even though an aneurysm in this artery was not evident on the noncontrast CT image acquired before the rupture. On the first day, bleeding persisted within the stomach wall, pain was irritating, and blood pressure was high, leading to arterial rupture. This case suggests that it is difficult to accurately predict the next vessel rupture in patients with SAM. In addition, arterial wall injuries were found in 6.7% of the cases where IRs were carried out.4 The arterial wall of SAM might be prone to dissection from mediolysis.4 Therefore, we support the present strategy of treating ruptured aneurysms only and conservatively monitoring other aneurysms. Additionally, upon encountering an abdominal hemorrhage without an aneurysm in the abdominal cavity, we must consider the possibility of SAM before aneurysm formation.
It was possible, if contrast CT had been carried out at the initial examination in our case, the findings of vascular irregularities could have led to SAM diagnosis. The patient could have been admitted and undergone appropriate conservative management, such as antihypertensive measures, to prevent rupture. Even if rupture had occurred, immediate intervention could have averted a fatal situation.
Although SAM results in intra-abdominal or gastrointestinal hemorrhage, no previous reports of SAM resulting in gastric wall hematoma are known. Segmental arterial mediolysis might need to be considered as a differential diagnosis for gastric wall hematomas of unknown cause.
CONCLUSION
We encountered a SAM that presented with a gastric intramural hematoma, with subsequent intra-abdominal hemorrhage on consecutive days without aneurysmal formation. In patients with SAM, we suggest a strategy that treats only ruptured aneurysms because it is difficult to accurately predict the next vessel to rupture.
ACKNOWLEDGMENTS
We would like to thank Editage for English language editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests for this article.
ETHICS STATEMENT
Approval of the research protocol: The study conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Informed consent: The patient provided informed consent for publication of this case report.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.




