Can Gram staining be a guiding tool for optimizing initial antimicrobial agents in bacterial infections?
Abstract
The emergence of multidrug-resistant organisms poses a significant threat to global public health, making the optimization of antimicrobial use crucial. Antimicrobial therapy is often initiated in emergency rooms (ERs) and intensive care units (ICUs), where patients are at high risk of infection. Prompt antimicrobial selection is essential in these facilities, and point-of-care testing can guide the appropriate initial antimicrobial therapy. Gram staining, a quick and inexpensive method, was previously used for point-of-care testing by physicians in the 1980s but was discontinued in 1988 in the United States. However, in Japan, the clinical practice of Gram stain-based antimicrobial therapy by physicians has continued in a limited number of hospitals. Several studies undertaken in Japan have shown that Gram staining carried out by trained physicians can reduce the overuse of broad-spectrum antimicrobial agents in ERs and ICUs without worsening patients' outcomes. Gram stain-based antimicrobial therapy reduced unnecessary use of carbapenems in the ER. Furthermore, Gram staining has been shown to significantly reduce the overuse of broad-spectrum antimicrobials without worsening clinical cure and mortality for patients with ventilator-associated pneumonia in the ICU. The classic technique of Gram staining has regained its usefulness through persistent clinical practice in Japan. It is hoped that Japanese researchers in this field will demonstrate to the world the efficacy of the classic technique of Gram staining in addressing this critical problem. Gram staining carried out by trained physicians could serve as a valuable means of optimizing antimicrobial treatment in ERs and ICUs.
INTRODUCTION
Over the past few decades, the incidence of serious infections due to multidrug-resistant organisms has increased, and these infections now constitute a serious threat to global public health.1, 2 However, development of new pharmaceutical agents has not been sufficient to solve this devastating problem.3, 4 Therefore, the importance of optimizing the use of antimicrobial agents has increased over the years.
The emergency room (ER) and the intensive care unit (ICU) are the most likely to initiate antimicrobial therapy because these patients are at increased risk of infection due to underlying conditions and exposure to multiple invasive devices.5, 6 Physicians are pressed to select antimicrobial agents promptly in these facilities because of the high urgency and severity. They often initiate broad-spectrum antimicrobial agents as an empirical treatment until the causative pathogens and antimicrobial susceptibility are identified. Although early broad-spectrum treatment helps to ensure that infections are treated effectively,7 overuse of broad-spectrum antimicrobial agents is driving antimicrobial resistance.8 These factors make it very difficult for physicians in ERs and ICUs to strike a balance between ensuring patient safety by using broad-spectrum antimicrobials and preventing further increases in drug-resistant bacteria by limiting the use of broad-spectrum antimicrobials. To solve these problems, point-of-care testing is essential to the guidance of appropriate initial antimicrobial therapy.
Gram staining, first described more than a century ago, is now one of the most important staining techniques in microbiology. It allows for a quick evaluation of the morphological characteristics of the infectious causative pathogens by staining a specimen collected from an infected site. As Gram staining is an inexpensive, simple examination used all over the world, it is thus potentially useful to guide appropriate initial antimicrobial therapy worldwide (Figure 1). This review aims to trace the history of Gram staining in clinical practice and to explore whether Gram staining could be a point-of-care testing technique for optimizing initial antimicrobial agents in patients with bacterial infections, based on recent clinical studies.
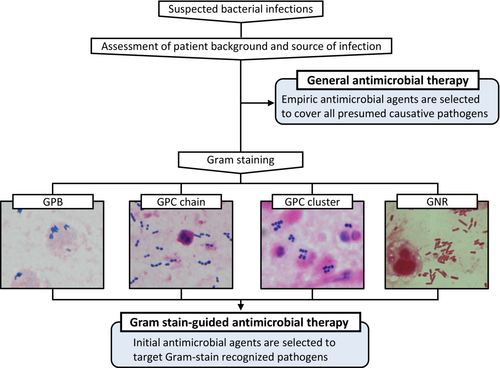
HISTORY OF GRAM STAINING IN CLINICAL PRACTICE
Named after Hans Christian Gram, who developed the method in 1884, the Gram stain allows one to distinguish between Gram-positive and Gram-negative bacteria on the basis of differential staining with a crystal violet-iodine complex and a safranin counterstain. Gram staining still ranks as one of the most important stains for bacteria. Because Gram staining was inexpensive and quick to carry out, by the 1980s there were several studies on the usefulness of Gram staining not only for microbiological testing but also for point-of-care testing by physicians for antimicrobial selection in patients with infectious diseases, especially pneumonia.9-11 However, Gram staining undertaken as a point-of-care testing method by physicians rapidly declined because of the Clinical Laboratory Improvement Amendments of 1988, which required that staff have credentials to interpret Gram stains of any specimens.12 In addition, the development of new antimicrobial agents has made it possible to provide initial empirical antimicrobial therapy with respiratory quinolones for a wide range of patients with community-acquired pneumonia, which could have had a small effect on the decline of Gram staining.12, 13 As a result, while 50% of general internists in the United States carried out Gram staining in their routine clinical practice in 1986, by 2004 this had dropped to 5%.14 In short, Gram staining as point-of-care testing by physicians has declined for reasons not dependent on adequate scientific validation of Gram staining.
In Japan, however, the clinical practice of Gram stain-based initial empirical antimicrobial therapy by physicians has been continued in a limited number of hospitals due to special circumstances. Okinawa Chubu Hospital provides postgraduate medical education that is affiliated with the University of Hawaii.15 The program started in 1966 when Okinawa was still controlled by the United States. At that time, Gram staining was also being performed by physicians in the United States, and the importance of Gram staining was emphasized. After Okinawa was returned to Japan in 1972, the program continued, and Gram staining has remained an important procedure in the clinical practice of infectious diseases performed by physicians at Okinawa Chubu Hospital. In fact, many studies on the usefulness of Gram staining have been reported by physicians at this hospital.16-20 Furthermore, postgraduate mandatory clinical residency training that began in Japan in 2004 has spread this concept to medical institutions other than Okinawa Chubu Hospital. As a result, Gram stain-based treatment by physicians has continued in Japan, albeit in only a few medical institutions.21 These situations led to a major divergence among Japan, North America and Europe: some physicians in Japan regard Gram staining as a physician's procedure, whereas those in North America and Europe think that Gram staining is no longer a physician's procedure.
WHAT IS REQUIRED FOR GRAM STAINING AS POINT-OF-CARE TESTING?
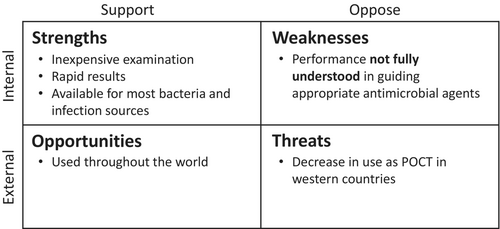
Several investigations have scrutinized the efficacy of Gram staining in bacterial infections with regard to its precision in determining the causative pathogens.22-25 Some research has indicated that the reliability of Gram staining in identifying the causative pathogens is moderate, leading to the conclusion that its utility as a predictor of antimicrobial selection in bacterial infections is limited.24, 25 However, what is imperative regarding Gram staining as a point-of-care test is not the aptitude to discern the causative pathogens but rather the capacity to discern the suitable spectrum of antimicrobial agents. Hence, when assessing the relevance of Gram staining as a point-of-care test, it is essential to consider the extent to which the antimicrobial selection based on Gram staining can correctly determine the appropriate antimicrobial agents (Figure 2). It is also crucial to weigh the potential impact of selected narrow-spectrum antimicrobial therapy on the patient's outcome. In this review, we examine the utility of Gram staining as a point-of-care test with respect to appropriate antimicrobial selection and patient outcomes.
GRAM STAINING AS A GUIDING TOOL FOR OPTIMIZING INITIAL ANTIMICROBIAL AGENTS IN ERs
The efficacy of Gram staining as point-of-care testing performed by physicians in the ER has been evaluated for infections commonly encountered in the ER, such as pneumonia,16, 17 urinary tract infections,17-19 and skin and soft tissue infections,17 and for meningitis,20 a serious infection (Table 1).
| Author, year | Department | Number of participants | Study design | Target diseases | Control antimicrobial selection | Antimicrobial selection | Clinical outcomes (intervention vs. control) | ||
|---|---|---|---|---|---|---|---|---|---|
| Appropriate antimicrobial selection | Clinical cure | Mortality | |||||||
| Fukuyama, 201416 | ER | 670 | Prospective |
Community-acquired pneumonia Healthcare-associated pneumonia |
Empirical therapy by physicians choice | 36.0% of patients with good-quality samples underwent pathogen-targeted therapya | NA | NA | 14/172 (8.1) vs. 45/498 (9.0) |
| Taniguchi, 201517 | ER | 208 | Prospective | Pneumonia, urinary tract infection, and skin & soft tissue infection | The JAID/JSC Guide to Clinical Management of Infectious Diseases 2011 | 50.0% reduction in anti-MRSA agents used and 92.0% reduction in antipseudomonal agents | 186/298 (89.4) vs. 191/208 (91.8) | NA | NA |
| Taniguchi, 202020 | ER | 34 | Retrospective | Bacterial meningitis | Japanese guidelines | 22.2% reduction in anti-MRSA agents used and 92.6% reduction in antipseudomonal agents | 34 (100) vs. 34 (100) | NA | NA |
| Taniguchi, 202219 | ER | 266 | Retrospective | Urinary tract infection | NA | 81.6% of patients used 1st- or 2nd-generation cephalosporins or ampicillin | 233/266 (87.6) | NA | 2 (0.8) |
| Yoshimura, 201726 | ICU | 131 | Retrospective | VAP | IDSA/ATS VAP guidelines 2005 | 55.9% reduction in anti-MRSA agents used and 26.1% reduction in antipseudomonal agents | 121/131 (92.4) vs. 125/131 (95.4) | NA | NA |
| Yoshimura, 201827 | ICU | 19 | Prospective | VAP | NA | 78.9% of patients used narrower-spectrum antimicrobials than recommended by IDSA/ATS VAP guidelines 2016 | NA | 13/19 (68.4) | NA |
| Yoshimura, 202228 | ICU | 206 | Multicenter RCT | VAP | IDSA/ATS VAP guidelines 2016 | 38.8% reduction in anti-MRSA agents used and 30.1% reduction in antipseudomonal agents | 89/103 (86.4) vs. 95/103 (92.2) | 79/103 (76.7) vs. 74/103 (71.8) | 14/103 (13.6) vs. 18/103 (17.5) |
- Abbreviations: ER, emergency room; ICU, intensive care unit; IDSA/ATS, Infectious Disease Society of America/American Thoracic Society; JAID/JSC, Japanese Association for Infectious Diseases/Japanese Society of Chemotherapy; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; RCT, randomized controlled trial; VAP, ventilator-associated pneumonia.
- a No data comparing selected antimicrobials in pathogens-targeted therapy versus empirical therapy.
Taniguchi et al.17 compared the selection of antimicrobials based on Gram staining by physicians to selection based on Japanese guideline recommendations for bacterial infections diagnosed in the ER. Their results indicated that Gram stain-based selection resulted in a reduction of broad-spectrum antimicrobial selection (such as antipseudomonal beta-lactams or anti-methicillin-resistant Staphylococcus aureus [MRSA] agents), as opposed to guideline-recommended selection (10/208 vs. 93/208). However, the selection rate of appropriate antimicrobials covering the causative pathogens was found to be comparable between the two groups: 89.4% (186/208) versus 91.8% (191/208). Another study assessed the efficacy of physician-performed Gram staining of cerebrospinal fluid for patients with meningitis.20 The authors noted that in cases of meningitis, Gram stain-based antimicrobial selection by physicians led to a decrease in carbapenem selection, without increasing inappropriate antimicrobial selection.
The efficacy of Gram stain-based antimicrobial therapy in ERs necessitates not just the selection of appropriate antimicrobial agents but also a comprehensive assessment of patient outcomes. Considering the pressing demands of ERs, incorporating Gram staining into the protocols could hinder prompt antimicrobial treatment, thus obviating the possibility of eliminating potentially unfavorable patient outcomes despite the judicious selection of antimicrobial agents. Fukuyama et al. undertook a study on the patient outcomes associated with Gram stain-based antimicrobial therapy.16 They found that patients who received narrow-spectrum antimicrobial therapy based on Gram staining experienced fewer initial treatment failures and had shorter duration of antimicrobial therapy and hospital stay in comparison to those treated with empiric broad-spectrum antimicrobial therapy.
All of the above studies indicated that Gram staining carried out by physicians as point-of-care testing can reduce the overuse of broad-spectrum antimicrobial agents without increasing initial treatment failure for patients with bacterial infections in ERs. Given the singular source of these studies, it cannot be assumed that equivalent procedures are universally accessible across all medical facilities. Nonetheless, Gram staining conducted by trained physicians could serve as a valuable means of optimizing antimicrobial treatment in ERs.
GRAM STAINING AS A GUIDING TOOL FOR OPTIMIZING INITIAL ANTIMICROBIAL AGENTS IN ICUS
The ICU is one of the largest consumers of antibiotics in the hospital. Several studies showed that 50%–70% of patients were considered to be infected and were treated with antibiotics during their ICU stay.6, 29, 30 In particular, pneumonia accounts for approximately half of all infections in ICUs and is therefore the most important disease requiring optimization of antimicrobial therapy in ICUs.6 The 2016 clinical practice guidelines for ventilator-associated pneumonia (VAP) published by the Infectious Disease Society of America (IDSA) and American Thoracic Society (ATS) recommend a broad-spectrum antimicrobial therapy covering both MRSA and Pseudomonas aeruginosa as an empirical treatment and state that the role of Gram staining in guiding empiric therapy is unclear.31 Herein, we summarize the effectiveness of Gram staining as a guiding tool for optimizing antimicrobial agents in patients with VAP, which was later reported subsequent to the publication of the guidelines.
A retrospective analysis was carried out to evaluate the impact of Gram staining on the initial antimicrobial selections of patients with VAP.26 The study compared two treatment algorithms, one based on Gram staining results and the other on the IDSA/ATS VAP guidelines. The results revealed that Gram stain-guided antimicrobial selections could significantly reduce the overuse of broad-spectrum antimicrobials without elevating the risk of inappropriate initial antimicrobial selections. However, this study was limited in that it only retrospectively simulated the appropriateness of antimicrobial selection, without evaluating actual patient outcomes when treated based on Gram staining. Consequently, a subsequent prospective interventional study was carried out to ascertain the efficacy of Gram stain-based antimicrobial therapy in curing pneumonia.27 The results of this study concurred with those of previous large clinical trials, affirming Gram stain-based antimicrobial therapy to be an effective cure for pneumonia. Prior studies had not evaluated whether patient outcomes such as clinical cure of pneumonia or 28-day mortality were worse compared to guideline-recommended therapy. In addition, it was necessary to confirm whether Gram staining, an intervention that is susceptible to the skill of the evaluator, would be equally useful at other institutions. Therefore, a multicenter, open-label, randomized, noninferiority trial named the GRACE-VAP trial was designed to investigate whether Gram stain-guided restricted initial antimicrobial therapy was noninferior to guideline-based broad-spectrum empirical therapy with respect to the clinical cure of VAP.32 The results of the GRACE-VAP trial showed that clinical cure achieved with Gram stain-guided antimicrobial therapy was noninferior to guideline-based antimicrobial therapy (risk difference, 0.049; 95% confidence interval, −0.07 to 0.17; p < 0.001 for noninferiority).28 There were also no significant differences between the groups in 28-day mortality, 28-day ICU-free days, or duration of antimicrobial therapy. Additionally, Gram stain-guided antimicrobial therapy reduced the use of antipseudomonal agents by 30.1% and anti-MRSA agents by 38.8% compared to guideline-based antimicrobial therapy.
These series of studies suggest that Gram stain-based antimicrobial therapy for critically ill patients with VAP reduces the use of broad-spectrum antimicrobials without worsening patient outcomes (Table 1).
OPERATION OF GRAM STAINING AS POINT-OF-CARE TESTING IN ERS AND ICUS
The results of the studies undertaken thus far suggest that antimicrobial therapy based on Gram staining can restrict the use of broad-spectrum antimicrobial agents without compromising patient outcomes, even in critical care settings such as ERs and ICUs. When implementing the system in clinical practice, certain considerations should be taken into account. First, it is worth noting that the studies on the efficacy of Gram staining were carried out at facilities with trained physicians. Therefore, it is advisable for physicians to collaborate with microbiology laboratories to obtain adequate training before initiating Gram staining. Osaka General Medical Center, the central institution that reported the usefulness of Gram staining in ICUs, installed a Gram staining system in the ICU in 2012. According to data from previous studies,26 Gram staining carried out by physicians in 2013 could safely limit the selection of broad-spectrum antimicrobial agents. Therefore, we believe that it will be feasible to safely limit the use of broad-spectrum antimicrobials within a year if a Gram staining system is installed in ERs and ICUs. Second, the selection of a specific antimicrobial agent based on the results of Gram staining is dependent on the regional antibiogram. For instance, the decision to use an anti-MRSA agent when Gram-positive cocci clusters are present is contingent on the MRSA to S. aureus ratio in the region. Bearing these points in mind, it is possible to implement a practice that safely limits broad-spectrum antimicrobials using Gram staining.
FUTURE PERSPECTIVE
The technique of Gram staining carried out by physicians, whose use had declined globally, has regained its usefulness through the results of studies carried out in a small yet persistent clinical practice in Japan. The situation concerning drug-resistant organisms has undergone significant change since the 1990s when Gram staining carried out by physicians was discontinued in the United States. Therefore, there is an unprecedented requirement for point-of-care testing to select appropriate antimicrobial agents. In light of the daunting issue of the increasing numbers of drug-resistant bacteria, it is hoped that Japanese researchers in this field will once again demonstrate to the world the efficacy of the classic technique of Gram staining in addressing this critical problem.
ACKNOWLEDGMENTS
We would like to thank Asako Matsushima (Clinical Department of Emergency Medicine, Nagoya City University Hospital) for sharing with us the detailed progress of the introduction of the Gram staining system at the Osaka General Medical Center.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this article.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




