A case of glyphosate poisoning with falsely elevated creatinine levels measured by dry chemistry methods
Abstract
Background
The ingestion of glyphosate herbicides can cause severe acute kidney injury. We present a case of glyphosate poisoning in which creatinine levels were falsely elevated when measured using a dry chemistry analyzer.
Case Presentation
An 83-year-old man ingested glyphosate herbicide during a suicide attempt and presented with gastrointestinal symptoms. A dry chemistry analyzer showed high creatinine levels (10.62 mg/dL); thus, the patient was transported to our hospital for renal replacement therapy. However, enzymatic testing showed a lower creatinine level (1.36 mg/dL). Further investigation comparing creatinine measurements revealed a high creatinine level only through the dry chemistry method, despite the relatively low glyphosate concentration (55 mg/L).
Conclusion
The dry chemistry method falsely shows an elevated creatinine value, which may affect clinical decisions regarding the management of patients with glyphosate poisoning. Careful interpretation of creatinine measurements using the dry chemistry method is important in glyphosate poisoning cases.
INTRODUCTION
Herbicide poisoning is a public health concern around the world. Approximately 14 million people worldwide die from intentional herbicide ingestion, and hundreds of thousands of people die from occupational or accidental poisoning. The World Health Organization has highlighted pesticides and herbicides as the primary means of suicide, noting that this is particularly prevalent in developing countries, where herbicides are often used in such incidents.1 Glyphosate-based herbicides are among the most widely used herbicides in agriculture worldwide, making glyphosate poisoning a common problem.
Glyphosate poisoning generally causes gastrointestinal symptoms, but at high doses, the symptoms can become severe, including respiratory distress, consciousness disorder, circulatory failure, kidney failure, and, ultimately, multi-organ failure. The fatality rate is 8% to 16%, making prompt intervention critical in severe cases.2 Notably, acute kidney injury (AKI) occurs in up to half of glyphosate poisoning cases and is associated with poor clinical outcomes,3 making renal function assessment essential in glyphosate poisoning cases.
Dry chemical analysis is a biochemical testing system that enables rapid and straightforward blood testing without the need for water or liquid reagents. Because of its ease of use, small equipment, and independence from water for measurement, this system is particularly useful in disaster situations and rural areas. However, this system is sensitive to the sample characteristics.4 As a result, there have been reports from external quality control surveys indicating potential discrepancies between this method and traditional liquid reagent methods.5 Here, we report a case of glyphosate poisoning in which the dry chemistry method yielded falsely elevated blood creatinine levels.
CASE PRESENTATION
An 83-year-old man attempted suicide by ingesting a glyphosate-based herbicide (containing 41% glyphosate-isopropylamine salt, 59% surfactant, and water). Approximately 4 h post-ingestion, he had abdominal pain, vomiting, and diarrhea, leading to a visit to a local clinic 6 h post-ingestion. The estimated ingestion volume was approximately 200 mL. At the clinic, he was alert, with the following vital signs: body temperature of 36.8°C, heart rate of 90 bpm, blood pressure of 168/85 mmHg, respiratory rate of 20/min, and an SpO2 of 96% in room air. He reported lower abdominal pain and nausea, with a flat abdomen and no tenderness.
A complete blood count and venous blood gas analysis revealed leukocytosis (white blood cell count, 41,000/μL) with no other significant abnormalities. The pH was 7.323, and the potassium level was 4.8 mEq/L, indicating mild acidemia without hyperkalemia. However, biochemical tests using the dry chemistry method (FUJI DRI-CHEM NX700® and FUJI DRI-CHEM SLIDE CRE-PIII®; FUJIFILM Corp., Tokyo, Japan) indicated a markedly elevated creatinine level of 10.62 mg/dL, suggestive of severe kidney injury. The physician considered the indication for renal replacement therapy and decided to transfer the patient to our hospital the same day.
At our facility (approximately 11 h post-ingestion), the patient's vital signs remained stable. The complete blood count and blood gas analysis showed no significant changes from previous results, with persistent findings of mild acidemia without hyperkalemia. Other test results, including chemistry and coagulation, showed no organ damage (Table 1). A repeat biochemical test using an enzymatic method (colorimetric) on the TOSHIBA TBA 2000FR® (TOSHIBA Corp., Tokyo, Japan) and the L-type Creatinine M reagent® (FUJIFILM Corp., Tokyo, Japan) revealed a creatinine level of 1.36 mg/dL, significantly lower than the prior result obtained at the initial clinic. Imaging studies revealed no significant findings. Considering the patient's stable systemic condition without any organ dysfunction, sufficient urine output, and balanced acid–base balance, we decided to hospitalize the patient and observe him without renal replacement therapy.
| Previous | Our | Normal range | |
|---|---|---|---|
| Complete blood count | |||
| White blood cell count (×103/μL) | 34 | 41 | 3.3–8.6 |
| Hemoglobin (g/dL) | 9.9 | 8.0 | 13.7–16.8 |
| Red blood cell count (×104/μL) | 327 | 261 | 407–610 |
| Platelet count (×104/μL) | 178 | 148 | 158–348 |
| Biochemistry test | |||
| Urea nitrogen (mg/dL) | 22.3 | 19.2 | 8.0–20.0 |
| Creatinine (mg/dL) | 10.62 | 1.36 | 0.65–1.07 |
| Aspartate aminotransferase (IU/L) | 15 | 15 | 13–30 |
| Alanine aminotransferase (IU/L) | 13 | 10 | 10–42 |
| Lactate dehydrogenase (IU/L) | 210 | 236 | 124–222 |
| Creatine kinase (U/L) | 111 | 111 | 59–248 |
| Coagulation test | |||
| Prothrombin time international normalized ratio (INR) | – | 1.25 | |
| Activated partial thromboplastin time (s) (gas analysis) | – | 39.7 | 25.0–35.0 |
| pH | – | 7.370 | 7.350–7.450 |
| pCO2 (mmHg) | – | 39.2 | 35.0–45.0 |
| pO2 (mmHg) | – | 71.0 | 75.0–110.0 |
| HCO3 (mEq/L) | – | 22.8 | 20.0–26.0 |
| Base excess | – | −2.3 | −3.0–3.0 |
| Lactate (mmol/L) | – | 0.4 | 0.5–2.0 |
After admission, the patient's renal function (creatinine level), urine output, and general condition remained stable. The patient was discharged on the 3rd day of hospitalization.
Further investigations were carried out to compare the creatinine values measured using dry chemistry and enzymatic methods. A blood sample collected from the patient in a serum separation tube 11 h post-ingestion was centrifuged, separated, and frozen until measurement. The serum was analyzed using the same dry chemistry method as well as another enzymatic method (amperometric) on the i-STAT® 1 analyzer with the i-STAT CREA CARTRIDGE® (Abbott, Lake County, IL, USA) for comparison. The dry chemistry method showed an elevated creatinine level of 5.60 mg/dL, which was higher than that of the other methods. Additionally, we measured blood glyphosate concentration using LC–MS/MS, which returned a value of 55 mg/L (Table 2).
| End substance for reaction | Method for measurement | Values (mg/L) | ||
|---|---|---|---|---|
| Glyphosate | 55 | |||
| Creatinine | Dry chemistrya | Ammonia | Colorimetric | 5.6 |
| Enzymatic (colorimetric)b | Hydrogen peroxide | Colorimetric | 1.36 | |
| Enzymatic (amperometric)c | Hydrogen peroxide | Amperometric | 1.41 |
- a FUJI DRI-CHEM NX700®and FUJI DRI-CHEM SLIDE CRE-PIII, FUJIFILM Corp., Tokyo, Japan.
- b TOSHIBA TBA 2000FR® TOSHIBA Corp., Tokyo, Japan, and L-type Creatinine M, FUJIFILM Corp., Tokyo, Japan.
- c i-STAT® 1-analyzer, i-STAT CREA CARTRIDGE, Abbott, Chicago, US.
DISCUSSION
Glyphosate is typically stabilized in salt forms, such as potassium salt or isopropylamine salt, and is marketed in combination with surfactants such as polyoxyethyleneamine (POEA),6 which are much more harmful than glyphosate itself.7 AKI is caused by the POEA-induced disruption of cell membranes, mitochondrial damage, and subsequent oxidative stress, leading to renal cell death. In addition, hypotension, metabolic acidosis, and systemic inflammatory responses associated with glyphosate poisoning can lead to acute tubular necrosis, resulting in AKI. Therefore, AKI associated with glyphosate poisoning is considered to be the result of multiple intertwined factors. The rapid initiation of renal replacement therapy significantly improves outcomes in patients with AKI.3
In this case, elevated creatinine levels were observed at the previous clinic using a dry chemistry method, and a parallel retest of the same sample confirmed an elevation only in the dry chemistry measurements. In the dry chemistry method, creatinine in samples is broken down by creatinine deiminase in the reaction layer. This releases ammonia gas, which reaches the detection layer and is colored by reacting with bromophenol blue. The color is measured using a spectrophotometer and converted to a creatinine concentration measurement.8 In this case, the glyphosate herbicide contained isopropylamine, an ammonia derivative, which caused a falsely high value (Figure 1). Furthermore, this effect persisted even when blood glyphosate levels were relatively low (approximately half a day after ingestion).
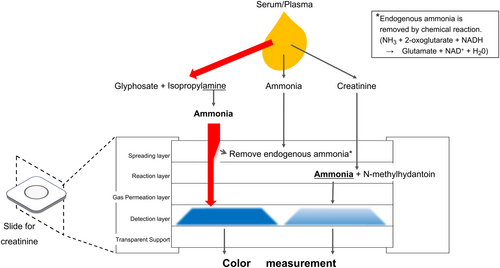
In contrast, the enzymatic method for creatinine measurement does not use ammonia.9 Therefore, glyphosate additives do not affect the creatinine results. In cases of ingestion of glyphosate herbicides containing isopropylamine salt, high creatinine values measured using a dry chemistry method should be interpreted carefully.
Similar to glyphosate additives, substances that generate ammonia in dry chemistry (such as flucytosine) cause a pseudo-elevation of creatinine in dry chemistry (Kodak Ektachem).10 In addition, the other methods, especially the Jaffe reaction, are interfered with by other chromogenic substrates; thus, these methods may show false elevations in patients with conditions that increase acetoacetate production (ketoacidosis) and when taking some medications (cefoxitin, cefazolin, barbiturates, etc.).10 The blood urea nitrogen (BUN)/creatinine ratio may be helpful in detecting the pseudo-elevation of creatinine; in our case, the BUN/creatinine ratio was very low at 2.2 when creatinine was measured by the dry chemistry method. Therefore, in case of unexpectedly high creatinine values, especially in relation to BUN, these influences should be considered, and a careful diagnosis should be made, including measurements with other methods.
CONCLUSION
The dry chemistry method can falsely yield high creatinine values. Careful diagnosis, including the use of alternative testing methods, is necessary to assess the development of AKI due to glyphosate poisoning.
ACKNOWLEDGMENTS
We thank the staff at the University of Miyazaki Hospital for their help in the management of this case. We also thank SHOKUKANKEN, Inc. (Maebashi City, Gunma, Japan) for their assistance in the measurement of serum glyphosate levels.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: Written consent was obtained from the patient's spouse for sample storage, measurement of creatinine and glyphosate levels, and publication.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this case report are openly available.




