Jesaconitine monitoring in a case of severe aconitum poisoning with torsade de pointes treated via extracorporeal membrane oxygenation
Abstract
Background
Aconitum poisoning can cause severe arrhythmias. We report, for the first time, the detailed blood and urine concentrations of four aconitine alkaloids in a male patient in his 20s who ingested aconite roots with suicidal intent.
Case Presentation
The patient developed refractory torsade de pointes (TdP) and required veno-arterial extracorporeal membrane oxygenation. His TdP resolved 7 h after arrival, with sinus rhythm returning within 12 h. The patient was discharged 6 days later. Subsequent measurements of the four alkaloids over time showed that jesaconitine had the highest serum concentration, with the patient's sinus rhythm returning when the jesaconitine concentration was less than 1 ng/mL.
Conclusion
This report provides valuable insights into the disposition of aconitine alkaloids during severe intoxication. The changes in jesaconitine concentrations over time correlate with clinical symptoms, suggesting that these levels could guide treatment decisions in patients with severe Aconitum poisoning.
BACKGROUND
Aconitum is a plant with a long history of use in traditional oriental medicine. In recent years, interest in its diverse pharmacological activities, such as antitumor, anti-inflammatory, and analgesic effects, has increased, and various studies are underway.1 Conversely, aconite is also widely traded as an ornamental plant because of its beautiful flowers, but its strong toxicity has occasionally caused overdose problems in individuals with suicidal intentions.
Aconitum contains toxic aconitine alkaloids that act primarily on sodium channels, and the four main toxic components of aconitine are aconitine, mesaconitine, jesaconitine, and hypaconitine.1 According to a review that compared the toxicity of aconitine by subcutaneous injection into mice, the toxicity of each aconitine was as follows: Jesaconitine (LD50 0.23 mg/kg) ≥ Mesaconitine (LD50 0.19–0.25 mg/kg) > Aconitine (LD50 0.24–0.55 mg/kg) > Hypaconitine (LD50 1.1–1.9 mg/kg).1 These alkaloids can cause vomiting, diarrhea, paresthesia, numbness, weakness, sweating, altered mental status, ventricular arrhythmia, bradycardia, and hypotension, which can lead to cardiac arrest.1-3
We present the case of a male in his 20s who developed refractory torsade de pointes (TdP) due to suicidal Aconitum ingestion and was successfully treated with veno-arterial extracorporeal membrane oxygenation (VA-ECMO). To the best of our knowledge, this is the first report of blood concentrations of four aconitines measured over time in a patient with severe Aconitum poisoning, and our findings highlight the possibility of using the four aconitine alkaloids blood concentrations, including jessaconitine, to evaluate clinical symptoms. Moreover, it was found that, to perform a toxicological analysis of aconite poisoning, it is unclear which aconitine alkaloids are the main toxic substances, and it is better to measure at least four aconitine alkaloids, including jessaconitine.
CASE PRESENTATION
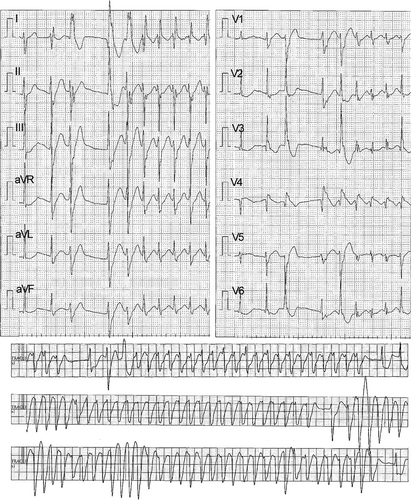
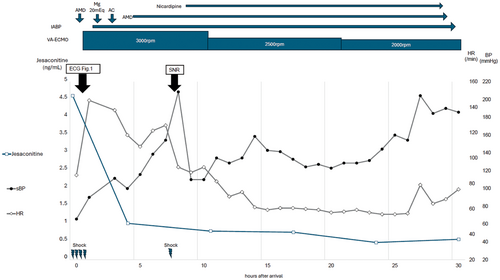
A male in his 20s with no prior medical history called emergency medical services, reporting “numbness.” Upon arrival at our emergency department, his initial assessment revealed the following: respiratory rate, 15 breaths/min; SpO2, 96% (room air); blood pressure, 85/66 mmHg; heart rate, 80 beats/min; Glasgow Coma Scale score (GCS), 3 (E1V1M1); pupil diameter, 4 mm/4 mm; contralateral light reflex, +/+; and body temperature, 36.4°C. Electrocardiogram (ECG) monitoring revealed a series of premature ventricular contractions (PVCs) and occasional convulsions. His level of consciousness temporarily recovered to E1V3M5 on the GCS, at which time he remarked “aconite,” which led to the diagnosis of Aconitum poisoning. Arterial blood gas analysis on admission revealed the following: pH, 7.365; PaO2, 79.8 mmHg; PaCO2, 39.0 mmHg; HCO3, 21.8 mmol /L; lactate, 2.99 mmol/L; metabolic acidosis; and hyperlactatemia. Whole-body computed tomography revealed no significant abnormalities. The patient's consciousness again declined to E1V1M1 on the GCS. ECG indicated that a series of PVCs led to TdP and an inability to palpate the carotid artery; thus, cardiopulmonary resuscitation was initiated (Figure 1). Multiple electrical defibrillations were performed, and amiodarone was administered; however, the TdP persisted. VA-ECMO was initiated 1 h after arrival due to refractory TdP. Coronary angiography revealed no significant stenosis in the coronary arteries. The patient received 50 g of activated charcoal, targeted temperature management therapy at 36°C was initiated, and he was admitted to the intensive care unit. After admission, the TdP persisted, and the patient remained dependent on VA-ECMO. Defibrillation was performed, thinking that blood concentration would decrease over time; this resulted in a self-paced heartbeat. The patient's sinus rhythm returned 12 h after arrival. Due to improved cardiac function and reduced PVCs, VA-ECMO was successfully discontinued on hospital day 2, 27 h after arrival (Figure 2). After his level of consciousness had improved, he told us that he had attempted suicide by consuming two Aconitum japonicum subsp. subcuneatum roots bought online, which he had cut and ingested (without cooking) 4 h before calling for emergency help. Since there were no neurological sequelae and the ECG waveforms had normalized, the patient was discharged on hospital day 6 and began visiting the psychiatric department.
LC–MS/MS analysis of biological samples
Sample extraction and LC–MS/MS analysis were performed with modifications to previously reported methods.2 Sample extraction was performed using the Micro Volume QuEChERS Kit (Shimadzu Corporation, Kyoto, Japan) and diazepam-d5 (Sigma-Aldrich Japan, Tokyo, Japan) as the internal standard. Biological samples were diluted as needed and extracted. A calibration curve was calculated using the aconitum diester alkaloids standard (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). Monitored ions were the precursor ion of m/z 646.3 to the production of m/z 105.0 for aconitine, m/z 676.25 to 135.15 for jesaconitine, m/z 632.30 to 105.05 for mesaconitine, m/z 616.20 to 556.45 for hypaconitine, and m/z 290.15 to 198.20 for diazepam-d5.
Measurement results
At the patient's arrival at the hospital, the blood concentrations of aconitine, jesaconitine, and mesaconitine were 0.35, 4.54, and 0.58 ng/mL, respectively, but hypaconitine was not detected (Table 1). Four hours later, only jesaconitine was detected, and the other aconitine alkaloids were not detected. The concentrations of aconitine, jesaconitine, mesaconitine, and hypaconitine in the urine at the time of admission were detected as 233.76, 1466.34, 450.29, and 50.87 ng/mL, respectively. Furthermore, the concentrations of aconitine, jesaconitine, mesaconitine, and hypaconitine in the gastric juice were 31.26, 184.86, 49.15, and 9.11 ng/mL, respectively (However, as the liquid part of the gastric juice was used as the sample without homogenizing the solid matter, there is a high possibility that the concentration will differ depending on the location of collection, so these are reference values).
| Hours from arrival | Concentration (ng/mL) | |||
|---|---|---|---|---|
| Aconitine | Jesaconitine | Mesaconitine | Hypaconitine | |
| Serum | ||||
| 0 | 0.35 | 4.54 | 0.58 | <LOQ3 |
| 4 | <LOQ1 | 0.94 | <LOQ2 | <LOQ3 |
| 10 | <LOQ1 | 0.72 | <LOQ2 | <LOQ3 |
| 16 | <LOQ1 | 0.69 | <LOQ2 | <LOQ3 |
| 22 | <LOQ1 | 0.4 | <LOQ2 | <LOQ3 |
| 28 | <LOQ1 | 0.49 | <LOQ2 | <LOQ3 |
| Urine | ||||
| 0 | 223.76 | 1466.34 | 450.29 | 50.87 |
| 4 | 23.87 | 208.23 | 19.2 | 3.38 |
| 10 | 21.2 | 215.04 | 18.52 | 3.23 |
| 16 | 7.12 | 62.53 | 3.88 | 0.9 |
| 22 | 3.49 | 32.66 | 1.37 | 0.41 |
| 28 | 0.44 | 3.92 | 0.3 | <LOQ4 |
| Stomach contentsa | ||||
| 0 | 31.26 | 184.86 | 49.15 | 9.11 |
- Note: A male in his 20s ingested aconite roots in a suicide attempt, developing refractory torsade de pointes requiring ECMO. Jesaconitine had the highest serum concentration, with sinus rhythm returning when its level dropped below 1 ng/mL. This case highlights the potential of alkaloid levels to guide Aconitum poisoning treatment.
- Abbreviation: LOQ, limit of quantification LOQ1 < 0.1, LOQ2 < 0.2, LOQ3 < 0.3, LOQ4 < 0.4.
- a Because the only liquid portion was used as a sample without homogenizing the solids in the gastric juice, the concentration is likely to vary depending on the collection site and is not quantifiable.
DISCUSSION
Aconitine often causes symptoms within 15–30 min of ingestion, with an estimated lethal dose of 3–6 mg.2 It is primarily metabolized in the liver, and after metabolism, it is excreted by the kidneys.1 The half-life of aconitine alkaloids during intoxication varies widely among individuals.4 A review of reports of serum alkaloid concentrations in patients with aconite poisoning indicated that maximum total concentrations of the four aconitines ranged from 0.7 to 6.4 ng/mL in survivors and from 15.1 to 498.1 ng/mL in nonsurvivors.5 The disappearance of arrhythmias related to serum alkaloid concentrations and their resolution occurs when serum alkaloid concentrations drop below 0.5–1.5 ng/mL.3 In this study, the concentrations of aconitine, jesaconitine, mesaconitine, and hypaconitine in the serum, urine, and gastric juice of the patient were measured during hospitalization. This report on the temporal changes in the concentrations of four aconitines in a patient whose condition was severe enough to warrant the use of VA-ECMO is valuable for the medical community. Serum jesaconitine concentration was the highest in this patient. Although the ratio of aconitine alkaloids can vary between strains and sites,6 it can be inferred that the Aconitum japonicum subsp. subcuneatum ingested by our patient had a high jesaconitine content. The concentrations of aconitine alkaloids in gastric juice were similar to those in urine in the following order: jesaconitine > mesaconitine > aconitine > hypaconitine. Few reports have measured the four highly toxic aconitines.4, 5, 7-9 While the concentration of aconitine has often been assessed in cases of Aconitum poisoning, jesaconitine is more toxic than aconitine. It is crucial to measure the total concentration of aconitine alkaloids, especially in cases like this, where jesaconitine is the primary toxic substance. In this case, the blood concentration of jesaconitine, which is not usually measured, was the highest, and it was identified as the main component causing the poisoning. If jesaconitine had not been measured, the blood concentrations of the other aconitine compounds would have been very low, and even if the patient had given a statement, it might not have been possible to prove that this case was aconite poisoning.
In the treatment of arrhythmias, defibrillation and antiarrhythmic drugs are generally considered ineffective. Some studies suggest that magnesium sulfate, hemodialysis, and direct hemoperfusion therapy may be effective; however, these approaches are not considered established treatments.5, 10 When the total blood concentration of aconitine fell below 1 ng/mL, the patient's normal sinus rhythm was restored through electrical defibrillation, allowing him to be weaned off VA-ECMO. This observation aligns with the course of aconitine concentration noted in existing reports.8 From past literature, including this study, the concentration of aconitine in cases where VA-ECMO was required was 1.41–5.47 ng/mL, and in cases where it was not required, it was 0.65–7.79 ng/mL, so it seems that the concentration of aconitine is not related to the introduction of VA-ECMO.3-5 Currently, it is not possible to measure blood concentrations immediately at the bedside; however, when such measurements become available, they could serve as guidelines for discontinuing VA-ECMO.
CONCLUSION
We measured blood and urine concentrations of the four aconitines over time in a patient with severe aconite poisoning. In cases of Aconitum poisoning, it is important to evaluate the concentration of aconitine and the total concentration of aconitine alkaloids. The blood concentrations were consistent with the clinical symptoms in our patient. These may thus serve as useful indicators for the treatment of patients with severe Aconitum poisoning.
CONFLICT OF INTEREST STATEMENT
Dr. Kenji Dohi is an Editorial Board member of AMS Journal and a co-author of this article. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. No other authors have any conflicts of interest to disclose.
ETHICS STATEMENT
Approval of the research protocol: N/A.
Informed consent: The patient has provided informed consent for the publication of his case history.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data pertaining to this case are contained within the article.




