Krüppel-like factor 4 transcription factor in blood–brain barrier endothelial cells: A potential role in Alzheimer's disease
Abstract
Alzheimer's disease is the most prevalent chronic neurodegenerative disorder worldwide, with no sufficient cure. Ongoing research is focused on developing new therapies aimed at preventing or delaying the onset of symptoms, slowing disease progression, and improving cognitive and behavioral outcomes in individuals affected by Alzheimer's disease. Among the various pathological changes associated with this condition, blood–brain barrier (BBB) leakage plays a crucial role as it serves as a vital boundary for maintaining central nervous system (CNS) health. Preserving the integrity and functionality of the BBB is essential to protect the brain from amyloid-β accumulation, neuroinflammation, and neuronal degeneration. This review summarizes models of Alzheimer's disease characterized by BBB leakage over time. More importantly, we introduce Krüppel-like factor 4 (KLF4), a transcription factor involved in vascular systems, and discuss its relevance to Alzheimer's disease. By elucidating the functions of KLF4 within both vascular and CNSs, this review highlights its potential role in modulating BBB integrity in Alzheimer's pathology, which may contribute to therapeutic strategies for managing this debilitating condition.
1 INTRODUCTION
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder and represents the leading form of dementia.1 The incidence of this condition is steadily increasing, largely attributable to global population aging.2 Between 1990 and 2019, both the incidence and prevalence of AD, along with other forms of dementia, increased by 147.95% and 160.84%, respectively.3 As of 2020, over 55 million individuals worldwide are living with dementia, and this number is expected to increase to 78 million by 2030 and 139 million by 2050, highlighting the growing global burden of AD.4 However, it is essential to recognize that current pharmacological treatments for AD do not halt or reverse its progression. Consequently, prioritizing strategies aimed at reducing the risk of developing dementia and identifying more effective therapeutic interventions are imperative.
Of all the risk factors, blood–brain barrier (BBB) leakage globally impacts the central nervous system (CNS). This leakage can lead to decreased amyloid-beta (Aβ) clearance, reduced glucose uptake in the brain, accumulation of neuroinflammation, and loss function of efflux transporters, among other consequences.5 The characteristics of BBB damage in AD comprise alterations in intercellular structures, diminished expression of transendothelial transporters, induction of vasoactive mediators, and activation of astrocytes as well as monocytes/macrophages. BBB dysfunction can be detected using magnetic resonance imaging of specific brain regions during the early stages of AD.6 Therefore, maintaining BBB integrity is crucial. In this review, we first reviewed the role of Krüppel-like factor 4 (KLF4) in the BBB and the CNS in the brain, and further addressed the possible mechanism of KLF4 in the BBB. We will focus on KLF4, an important transcription factor in various cellular pathways, particularly concerning endothelial cell function. By examining the role of KLF4 in the vascular system, we hope that the function of KLF4 in the BBB can provide new ideas for the treatment of AD.
2 OVERVIEW OF AD ANIMAL MODELS WITH BBB LEAKAGES
The BBB is a highly selective barrier that forms between brain tissue and systemic circulation.7 Composed of brain capillary endothelial cells, pericytes, and astrocyte end feet, the BBB governs substance exchange while protecting the brain from pathogen infections and harmful agents. Its role in maintaining normal physiological states within the CNS holds significant biological importance.8
Animal models of AD are essential for understanding the pathogenesis of the disease, identifying biomarkers, and developing novel therapeutic strategies.9 Currently available animal models include genetically modified transgenic models, chemically induced models, naturally occurring models, and emerging model systems.10 Of these, transgenic models are developed using genetic engineering techniques that introduce AD-related genes (e.g., APP, PSEN1, PSEN2, and tau) into mice, rats, or other animals to mimic specific pathological features of AD. For instance, a study conducted by Tsuyoshi Nakai et al.11 in 2021 utilized a touch-screen basic task involving APP in a mouse model to enhance evaluation methods for early-stage candidate therapies for AD. Chemically induced models employ substances such as Aβ fragments, okadaic acid, or colchicine to induce neuropathological changes resembling those seen in AD within animal subjects. For example, streptozotocin is frequently used as a chemical inducer across various species exhibiting AD-like pathology.12 Conversely, naturally occurring models develop dementia symptoms through natural aging processes observed in nonhuman primates and dogs.13 These naturally occurring models are regarded as valuable resources for advancing research into AD due to their authentic pathogenic characteristics.14 However, each model possesses distinct advantages and limitations. Transgenic models, for instance, allow for the examination of specific gene functions under controlled conditions. But they may not fully replicate all pathological features of human AD. Chemical-induced models can rapidly induce AD-like pathology but often fail to exhibit the progressive nature characteristic of the disease. Naturally occurring models provide an environment that closely resembles human disease. Nevertheless, their study is expensive, and controlling for variables can be challenging.
Within the barrier, endothelial cells serve as the initial protective layer and play a crucial role in neurodegenerative diseases, particularly in the progression of AD. In the early stages of AD, endothelial cells exhibit reduced expression of tight junctions, which compromises BBB functionality. This disruption leads to alterations in the intracranial environment that exacerbate associated pathological changes observed in AD.15 Furthermore, endothelial cells within the BBB during AD may express increased levels of cell adhesion molecules and release pro-inflammatory mediators that further disrupt its integrity.16 Therefore, implementing treatments aimed at reducing early-stage endothelial inflammation could effectively mitigate neurodegenerative changes.17 In addition, as a component of the BBB, pericytes are closely arranged around endothelial cells and play a crucial role in maintaining the integrity of the BBB by secreting platelet-derived growth factor B.18 Pericyte-deficient mice exhibit vascular damage, degenerative changes in neurons, impairments in learning and memory, and the activation of neuroinflammatory responses.19 Numerous studies have demonstrated a strong correlation between BBB disorders and the pathological characteristics of AD. These characteristics include the accumulation of amyloid protein (Aβ), abnormal phosphorylation of tau protein, and neuroinflammation.20 Both living human brain studies and postmortem tissue analyses have shown that BBB breakdown occurs in AD. Additionally, multiple lines of evidence from animal models have indicated BBB disruption through the detection of Immunoglobulin G (IgG), Evans blue dye extravasation, dextran leakage, and loss of tight junctions in endothelial cells21 (Table 1). Furthermore, certain animal models, such as the APP/PS1 transgenic mouse, have demonstrated BBB breakdown occurring as early as 5 months of age. This occurrence takes place prior to the emergence of both AD pathological features and behavioral changes.22 The intriguing finding enriches our understanding and underscores the importance of studying animal models to elucidate the progression and onset mechanisms associated with AD.23 The aim of this review is to focus on the role played by endothelial cells within the BBB, which serves as a primary protective layer. By comprehending these underlying mechanisms, there exists potential for developing therapeutic strategies aimed at targeting the BBB to potentially slow or halt disease progression. Notably, KLF4, a zinc-finger transcription factor, plays a critical role in vascular development and maintenance. KLF4 affects the function of endothelial cells and vascular smooth muscle cells (SMC), thereby promoting blood vessel formation and maintaining the stability of its structure.
| Species | Manipulation | Findings | Earliest age with BBB leakages | Region | Reference |
|---|---|---|---|---|---|
| Mouse | APP | IgG, fibrin perivascular deposits; BBB leakage of Evans blue; loss of BBB tight junctions | 1 month | Cortex, hippocampus, thalamus | 24, 25 |
| PS1 | Microhemorrhages; endothelial degeneration | 10 months | Cortex, hippocampus | 24, 25 | |
| APP/PS1 | Microhemorrhages, albumin, IgG, fibrin perivascular deposits; endothelial degeneration; loss of BBB tight junctions | 4–9 months | Cortex, hippocampus, thalamus | 24, 25 | |
| 3× Tg (APP/PS1/tau) | DCE-MRI leakage; IgG protein levels increase | 4 months | Basement membrane, hippocampus | 24, 25 | |
| 5× Tg | IgG perivascular deposits; albumin-positive microvessels decrease | 4 months | Cortex | 24, 25 | |
| TR-APOE4 | BBB leakage of Evans blue; IgG perivascular deposits; loss of BBB tight junctions | 2 weeks | Hippocampus, cerebellum, spinal cord, cortex | 26, 27 | |
| Rat | APP | DCE-MRI leakage; loss of BBB tight junctions | 17 months | Hippocampus, cortex | 14, 28 |
| APP/PS1 | BBB leakage of Evans blue; loss of BBB tight junctions | 12 months | Frontal cortex | 29 | |
| Zebrafish | APP | Undetected | – | – | 30 |
| PS1 | Undetected | – | – | 31 | |
| MAPT(tau) | Undetected | – | – | 32 | |
|
NHP |
Spontaneous | Fibrin perivascular deposits | 5–25 years | Frontal, temporal, parietal cortices, cerebrovascular | 33-35 |
| Aβ oligomers injection | Fibrin perivascular deposits | 4 weeks to 5 months | Frontal cortex, hippocampus | 36 | |
| MAPT(tau) | Vascular basement membrane thickening and abnormal vascular morphology | After 6–10 weeks of hTau overexpression | Blood vessels in the hippocampus | 37 | |
| Canine | Spontaneous | Changes in the capillaries of the white matter, laminin immunoreactivity | 8–15 years | Prefrontal cortex, hippocampus | 14, 38, 39 |
- Abbreviations: Aβ, amyloid-beta; BBB, blood–brain barrier; DCE, Diffusion-Weighted Contrast; MRI, magnetic resonance imaging; NHP, nonhuman primate.
3 KLF4 IN VASCULAR DEVELOPMENT AND MAINTENANCE
Numerous genes are implicated in both BBB development and dysfunction. And one of them is KLF4. In contrast to other isoforms in the KLF4 family, KLF4 is a ubiquitously expressed transcription factor that functions in a variety of cellular processes, including cell growth, differentiation, and apoptosis. There is increasing evidence that KLF4 is potentially regulated in the neurophysiological and neuropathological processes of AD.40 KLF4 plays a critical role in maintaining endothelial cell integrity while also facilitating their differentiation. As a transcription factor, it regulates various cellular processes essential for proper vascular function, such as proliferation, differentiation, and embryonic development. In the field of reprogramming, KLF4 is particularly well known for its role in the induction of induced pluripotent stem cells.41 In patients, serum concentrations of KLF4 increase 48 h after the onset of ischemia compared to control groups. However, when moderate-to-severe stroke patients are compared with those who experienced minor strokes, serum levels of KLF4 were significantly lower at 48 h post-ischemic stroke onset.42 Clinical data indicate a complex role for KLF4. Exploring the function of KLF4 in vascular disease could yield valuable insights into vascular-related conditions.
3.1 Manipulation of KLF4 in the vascular system
KLF4 plays a crucial role in various cellular processes. Mice with a knockout of the Klf4 gene exhibit high mortality shortly after birth.43 Therefore, it is essential to produce specific knockouts for the investigation of KLF4 function. Vascular SMCs play a critical role in cardiovascular system development. To investigate their significance, specific knockout mice lacking KLF4 were produced using the SM22α promoter. These mice gradually died after birth, and some surviving individuals exhibited stunted growth. Additionally, these mice exhibited a significant decrease in cardiac output and reduced expression levels of cardiac genes such as Gata4.44 KLF4 not only contributes to cardiovascular development but also plays an essential role in maintaining normal vascular integrity and promoting neovascularization during tissue repair processes. Studies have shown that during the process of cardiac remodeling after ischemic reperfusion injury, KLF4 is activated and expressed in arterial SMCs and plays a key role in regulating the phenotypic transformation of SMCs, which is important for cardiac remodeling.45 Typically, KLF4 is not expressed in SMCs but is activated after vascular injury. However, it is expressed in endothelial cells in normal condition. In a study using KLF4 conditional knockout mice, produced by crossing Tie2-CRE mice with Klf4 floxed mice, an upregulation of cell adhesion molecules, such as vascular cell adhesion molecule-1 and e-selectin, was observed.46 In adult animals, KLF4 collaborates with KLF2 to maintain vascular integrity effectively. To investigate this further, adult mouse endothelial cells were genetically manipulated to specifically delete both KLF2 and KLF4 genes. This double-gene deletion resulted in the animals experiencing mortality starting on day 6 after tamoxifen administration. These findings clearly demonstrate that the absence of these two genes within endothelial cells leads to compromised vascular integrity and severe abnormalities within the blood coagulation system.47 Additionally, knockdown of KLF4 in human pulmonary microvessel endothelial cells48 as well as glioma endothelial cells49 exhibited increased transendothelial permeability. In summary, if KLF4 is deleted or suppressed in either SMCs or endothelial cells of the vascular system, it would result in heightened vascular leakage. Conversely, when expressed appropriately, KLF4 serves as a critical safeguard for maintaining vascular integrity.
3.2 KLF4 in endothelial differentiation and angiogenesis
KLF4 plays a crucial role not only in the development and maintenance of the vascular system but also in endothelial differentiation in vitro and angiogenesis. In the differentiation process of endothelial precursor cells (EPC) derived from bone marrow, overexpression of KLF4 promotes EPC differentiation, whereas silencing KLF4 impedes this process.45 This indicates that KLF4 may serve as a promising target for enhancing vascular repair and regeneration. Furthermore, it has been demonstrated that upregulation of KLF4 can enhance the differentiation of early-stage EPCs into late-stage EPCs, either synergistically or independently when combined with apelin-13 in cultures of primary EPCs from rats.50 During angiogenesis, KLF4 inhibits the expression of Delta-like 4 (DLL4) by interfering with the binding of the RBP-J-NICD-MAML complex to intron 3 of the Notch ligand DLL4. This regulatory function contributes significantly to overall angiogenic processes.51 In mouse models of retinal angiogenesis, specific overexpression of KLF4 resulted in increased blood vessel density and branching. Over time, these excessively formed vessels underwent substantial remodeling and ultimately transformed into normal retinal blood vessels in adult mice.52 Additionally, KLF4 exhibited effects comparable to those observed with DLL4 inhibition in oxygen-induced retinopathy models. It effectively reduced vascular occlusion and diminished neovascularization cluster formation.51 In zebrafish models, KLF4 has been identified to play a pivotal role in both angiogenesis and branch formation through its regulation of gene expression via binding to specific sites on the Cx40 promoter.53 Interestingly, within a mouse tumor model context, overexpression of KLF4 specifically within endothelial cells was found to inhibit tumor growth by inducing ineffective angiogenesis.54 These findings suggest that KLF4 may play a protective role even during tumor formation. Overall, KLF4 plays a significant role in the differentiation of endothelial cells and promotes angiogenesis in a protective manner.
3.3 KLF4 in endothelial tight-junction expression
The endothelial cells, constituting the first layer of the BBB, primarily protect the brain through tight junctions formed between adjacent cells. Numerous studies have demonstrated KLF4's involvement in regulating tight-junction expression.
VE-cadherin is an essential component of adhesion junctions within endothelial cells. The expression and activity of KLF4 directly influence VE-cadherin levels, which subsequently affect both the integrity of adhesion junctions and the barrier function of endothelial cells. This process is crucial for maintaining vascular integrity and preventing inflammatory factor infiltration.48 The mechanism by which KLF4 regulates VE-cadherin expression primarily involves its interaction with specific DNA sequences located upstream of the transcription start site within the vascular endothelial cadherin (VE-cadherin) promoter. This interaction is vital for modulating VE-cadherin expression at adhesive junctions.48
KLF4 expression was downregulated using KLF4 small interfering RNA (siRNA) in the immortalized bEnd3 cell line, a mouse brain endothelial cell line. Results indicated a significant reduction in tight-junction proteins such as ZO-1 and Claudin5.42 Similarly, it has been shown that KLF4 enhances promoter activities by interacting with “CACCC” DNA sequences found in the promoters of ZO-1, Occludin, and Claudin5, which are key components of BBB endothelial cell tight junctions.49 Another study reported that Tongxinluo, a traditional Chinese medicine, provides protection against hypoxia-induced disruption of tight junctions in human cardiac microvascular endothelial cells. This protective effect is mediated through promoting phosphorylation of KLF4 and inducing the expression of tight-junction proteins.55 In addition to its role in endothelial cells, KLF4 is involved in the regulation of tight-junction protein ZO-1 in ovarian cancer cells, Sertoli cells, and corneal epithelial cells, either independently or cooperatively.56-58 In summary, KLF4's interaction with the promoters of tight junctions can enhance their expression.
3.4 KLF4 in endothelial inflammation
Inflammatory factors have the potential to induce endothelial dysfunction, resulting in pro-adhesion and pro-thrombotic characteristics within endothelial cells. The expression level of KLF4 significantly influences the anti-inflammatory properties of these cells. Research has demonstrated that KLF4 exerts its anti-inflammatory effects by modulating the levels of various anti-inflammatory factors within endothelial cells. One notable example is KLF4's ability to enhance the expression of endothelial nitric oxide synthase (eNOS), a critical enzyme involved in nitric oxide (NO) synthesis. NO serves as an anti-inflammatory molecule with vasodilatory effects that contribute to maintaining barrier function and preserving the anti-inflammatory properties of the vascular endothelium.59 During endothelium–interstitial transition (EndMT), KLF4 plays a pivotal role in transforming endothelial cells. Studies indicate that decreased expression of KLF4 correlates with increased levels of α-smooth muscle actin (α-SMA), a marker indicative of endothelial-to-mesenchymal transition. Conversely, elevated levels of KLF4 help maintain the mature phenotype characteristic of healthy endothelial cells. Furthermore, KLF4 inhibits EndMT by influencing the eNOS/NO signaling axis, thereby protecting endothelial cells from inflammatory damage.60 In mouse models, overexpression of KLF4 results in heightened expression levels of various anti-inflammatory and antithrombotic factors such as eNOS and thrombomodulin. In contrast, knockdown experiments involving KLF4 lead to increased expression levels of vascular cell adhesion molecule-1 and tissue factor induced by tumor necrosis factor α (TNF-α).61 These findings suggest that KLF4 plays a crucial role in maintaining vascular health and may possess therapeutic potential for related diseases, due to its anti-inflammatory and antithrombotic properties. In zebrafish models, the application of lysine-specific demethylase 1 (LSD1) inhibitors has been shown to impact vascular endothelial cells significantly. Studies have reported that LSD1 inhibitors can effectively reduce the release of inflammatory cytokines IL-8 and IL-6 although simultaneously enhancing NO production in vascular endothelial cells. This effect is attributed to the increased expression of KLF2 and KLF4 proteins.62 Notably, deletion of KLF4 in conditional knockout mice exacerbated neointima formation after vascular injury. Conversely, overexpression of KLF4 was found to diminish the recruitment and proliferation of inflammatory cells. Further analysis conducted on cultured endothelial cells demonstrated that KLF4 inhibits TNF-α-induced expression of Vcam1 by obstructing nuclear factor-κB (NF-κB) binding to the Vcam1 promoter.46 Subsequent studies revealed that long noncoding RNA (lncRNA) NKILA, a key inhibitor of endothelial inflammation, regulates this process through an NF-κB/KLF4-positive feedback loop. Specifically, NKILA enhances the expression of the anti-inflammatory protective factor KLF4 in endothelial cells via a DNA methylation mechanism mediated by NF-κB.62, 63 Moreover, it has been discovered that KLF4 can inhibit NF-κB's transcriptional activity, thereby establishing an NKILA-controlled positive feedback loop.64 These findings indicate that KLF4 primarily functions as an anti-inflammatory agent within endothelial cells, safeguarding them from inflammation. In addition, KLF4 is widely expressed in a variety of tissues and plays a key regulatory role in the neurophysiological and neuropathological processes of AD.65
4 KLF4 IN ALZHEIMER'S DISEASE
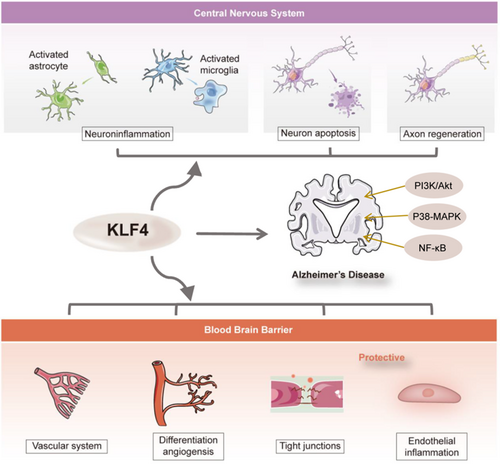
Recent studies have also suggested that KLF4 may play a significant role in the pathogenesis of AD through multiple pathways. It exhibits regulatory effects on neuroinflammation, neuronal apoptosis, axon regeneration, and antioxidant response. As previously mentioned, KLF4 plays an important role in BBB breakdown, which is also critical for AD development. In addition to its role in the BBB pathway, KLF4 influences AD through mechanisms that directly affect the CNS.
4.1 KLF4 in microglia function of neuroinflammation
Neuroinflammation is a key characteristic in the progression of AD.66 Research indicates that KLF4 can regulate neuroinflammatory responses associated with AD, extending beyond endothelial inflammation.67 In endothelial cells, KLF4 acts as an anti-inflammatory factor. However, studies have demonstrated that Aβ42 oligomers can enhance the expression of KLF4 in BV2 microglial cells and in brain microglia from AD mouse models.66 Silencing KLF4 has been shown to reduce Aβ42-induced neuroinflammation, whereas overexpression of KLF4 exacerbates this inflammatory response.67 In mice stimulated with lipopolysaccharide, an increase in KLF4 expression was observed within BV2 microglial cells. Furthermore, knocking down KLF4 resulted in decreased production of pro-inflammatory cytokines.68 Both in vivo and in vitro studies have established that IL-1β upregulates KLF4 via activation of the PI3K/Akt signaling pathway. This upregulation subsequently leads to positive regulation of endogenous IL-1β production as well as other inflammatory markers.69 Additionally, it has been found that KLF4 negatively regulates NOS expression and plays a significant role in modulating microglial immunological activity.70 Conversely, miR-25 802, a microRNA elevated in the plasma of AD patients, can directly bind to KLF4 mRNA and suppress its expression. Overexpression of miR-25 802 worsens AD progression by enhancing microglial activation through the NF-κB pathway, which is mediated by inhibiting KLF4.71 Consequently, it is suggested that upregulating KLF4 may serve as a potential strategy for mitigating cognitive deficits and neuroinflammation observed during the pathological process of AD. Therefore, further investigation into the precise role of KLF4 in neuroinflammation is warranted.
4.2 KLF4 in astrocyte function
Astrocytes perform numerous functions that contribute to overall brain homeostasis, assist in neurogenesis, determine the micro-architecture of gray matter, and defend the brain through evolutionarily conserved astrogliosis programs.72 They are also the primary cell type involved in neuroinflammation. Classically activated astrocytes (A1 subtype) exert neurotoxic effects by releasing pro-inflammatory mediators, whereas alternatively activated astrocytes (A2 subtype) provide neuroprotective effects through the secretion of anti-inflammatory mediators.73 After acute ischemic stroke in mouse models, KLF4 expression is upregulated in astrocytes, and KLF4 has been shown to exert a protective effect on cerebral vessels against ischemic injury. Results indicate that astrocytic KLF4 inhibits the activation of A1-type astrocytes by regulating NF-κB expression and promoting the polarization of A2-type astrocytes.73 Similarly, in rat models of ischemic injury, KLF4 expression is also upregulated in reactive astrocytes but remains undetected in resting ones.74 Moreover, ionizing radiation–induced DNA damage in astrocytes leads to an increase in KLF4 expression. Inhibition of KLF4 expression via lentiviral transduction results in fewer double-strand DNA breaks and an increase in single-strand DNA breaks.75 Beyond directly interfering with KLF4 within astrocytes, overexpression of KLF4 within neurons can influence brain-derived neurotrophic factor release from astrocytes. This subsequently promotes neuronal synapse formation and enhances resistance to oxidative stress.76 It indicates that KLF4 expressed either within or outside of astrocytes could modulate their function and consequently affect neuronal activity.
4.3 KLF4 in neuronal apoptosis
In addition to Aβ and tau pathology, patients with AD exhibit synaptic abnormalities, neuronal loss, cognitive decline, and memory impairments as the disease progresses.77 Furthermore, KLF4 influences gene expression related to apoptosis and plays a significant role throughout this process. In retinal ganglion cells (RGC) treated with H2O2, it has been demonstrated that apoptosis of RGCs increases through p53-dependent mechanisms, after the upregulation of KLF4. Additionally, overexpression of KLF4 in RGCs induces apoptosis in these cells.78 On the contrary, investigations into the effects and mechanisms underlying neuronal apoptosis induced by traumatic brain injury have revealed that sevoflurane can inhibit this process via activation of the p38-MAPK signaling pathway. This process involves targeting KLF4 to suppress its transcription, which subsequently reduces BBB permeability, decreases brain water content, and mitigates brain injury and neuronal apoptosis, ultimately enhancing neural function.79 Early brain injury (EBI) is a secondary complication that results shortly after subarachnoid hemorrhage (SAH), associated with elevated rates of disability and mortality. Recent findings indicate that miR-26b modulates the KLF4/STAT3/HMGB1 axis by targeting KLF4 to negatively regulate its expression. This mechanism exacerbates EBI and inflammatory responses in a rat model of SAH although promoting hemoglobin-induced astrocyte apoptosis. Experimental deletion of KLF4 has been shown to upregulate HMGB1, further aggravating EBI after SAH.80 The role of KLF4 in neuronal and astrocyte apoptosis remains controversial. Thus, it is challenging to definitively classify KLF4 as either a protective or detrimental factor in CNS apoptosis.
4.4 KLF4 in promoting axon regeneration
The phenomenon of neuron apoptosis is well established within neuroscience literature as a common feature associated with neurodegenerative diseases leading ultimately to neuronal death.81 Some studies suggest that KLF4 may facilitate post-injury repair and regeneration within the nervous system.82 To illustrate, researchers investigating the potential for restoring vision by reprogramming youthful epigenetic information discovered that the expression of Oct4, Sox2, and KLF4 genes in mouse RGCs within an ecological context led to the restoration of youthful DNA methylation patterns and transcriptomes. This process subsequently promoted axon regeneration after injury. Furthermore, a reversal of vision loss was observed in mouse models of glaucoma as well as in older mice.83 The aim was to determine whether targeted expression of the KLF4 gene could facilitate the regeneration of RGCs. It was demonstrated that overexpression of KLF4 can induce the transformation of retinal progenitor cells into RGCs. Notably, these newly produced RGCs are capable of migrating to their appropriate levels and projecting axons toward the optic nerve head.84
Nevertheless, knockdown experiments have substantiated that KLF4 serves as a principal inhibitory factor governing axon regeneration. In an optic nerve crush model, overexpression of miR-25-3p has been shown to promote axon regeneration and concomitantly downregulate KLF4.85 Using comprehensive miRNome-wide functional screening, researchers identified that silencing KLF4 along with miR-135a and miR-135b significantly enhances axon growth and cortical neuron migration in adult mice, both in vivo and in vitro. Moreover, vitreous injection of miR-135 s has been shown to facilitate RGC regeneration after optic nerve injury by inhibiting KLF4 activity. Conversely, depletion of miR-135s results in reduced axon regeneration.86 Additionally, whereas KLF4 is expressed in neural stem cells, its expression is significantly diminished in differentiated neurons. Sustained activation of KLF4 leads to impaired migration and development into glial cells among neuroprecursor populations. Notably, downregulation of KLF4 has been shown to significantly promote the transformation of radial and neonatal migrating neurons from a multipolar to a bipolar morphology.87 The role of KLF4 in axon regeneration remains contentious, particularly considering its potential involvement in processes such as neuronal inflammation and apoptosis. Overall, KLF4 plays a crucial regulatory role in various pathological processes of AD, and it is regarded as a potential therapeutic target for neurodegenerative diseases. By modulating the expression or activity of KLF4, new strategies and methods for treating AD may be provided.
5 THE THERAPEUTIC APPROACHES OF KLF4 IN MODULATING AD
The role of KLF4 in regulating AD conditions is multifaceted, involving multiple aspects of neuroinflammation, oxidative stress, apoptosis, and interaction with noncoding RNA. In the cerebellum of the 2× Tg-AD mouse model, which recapitulates the early-stage manifestations of AD, a significant reduction in the expression of the anti-inflammatory transcription factor KLF4 and a concomitant elevation in the expression of the inflammatory cytokine TNF-α were meticulously detected.88 This compelling evidence strongly implies that Aβ pathology has the potential to disrupt the normal function of α7-nicotinic acetylcholine receptors, consequently setting off a series of events that lead to the activation of the inflammatory response. As a result, the overexpression of KLF4 emerges as a potentially viable and promising approach for the intervention of early-stage AD. Accumulating studies have unambiguously demonstrated that lncRNA NKILA plays a pivotal and indispensable regulatory role in orchestrating pro-inflammatory responses. Specifically, NKILA positively regulates the expression of the anti-inflammatory protective factor KLF4 in endothelial cells through the well-characterized NF-κB-mediated DNA methylation mechanism.63 In a murine model, researchers have made the seminal discovery that KLF4, in concert with KLF2, effectively counteracts a diverse spectrum of vascular diseases, with atherosclerosis being a prime example, by precisely modulating the expression of anti-inflammatory and antithrombotic protective genes within endothelial cells.62, 89 Collectively, these results provide irrefutable evidence for the anti-inflammatory properties of KLF4 in the context of neuroinflammation, thereby furnishing novel and profound insights into the underlying pathogenesis of early-stage AD. These groundbreaking findings not only provide an in-depth fundamental understanding of the disease mechanism but also present highly promising potential targets for the rational design and development of innovative therapeutic strategies centered around KLF4. One such strategy is the treatment of AD via the overexpression of KLF4. For AD patients, adeno-associated virus (AAV) can be strategically engineered to specifically target and transduce vascular endothelial cells, thereby achieving the overexpression of KLF4 as a viable therapeutic intervention. Moreover, an alternative approach involves encapsulating KLF4 within nanoparticles, enabling the targeted overexpression of KLF4 in vascular endothelial cells, which holds great promise for the treatment of AD.
6 CONCLUSION
After nearly two decades of intense pharmacological research and drug development, no new therapies have emerged in AD. Still a large number of drugs are under review. In the 2024 AD drug development pipeline, combination therapies aimed at enhancing penetration of the BBB are proposed.90 This highlights the important role of BBB in AD. Our review emphasized the contribution of the transcription factor KLF4 to maintaining BBB integrity. It serves as a protective factor for BBB integrity. However, in other CNS cell types, like neuron, astrocytes, and microglia, KLF4 exhibits an ambiguous role (Figure 1). Therefore, specifically targeting KLF4 within endothelial cells rather than other CNS cell types may represent a promising therapeutic strategy for AD.
AUTHOR CONTRIBUTIONS
Ziying Wei: Investigation; validation; writing – original draft. Chunhua Liu: Validation; writing – review and editing. Jianyu Chen: Validation; writing – review and editing. Yuxiao Yao: Funding acquisition; supervision; validation; writing – review and editing. Dajiang Qin: Funding acquisition; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
None.
FUNDING INFORMATION
This work was supported by the Key Laboratory of Guangdong Higher Education Institutes (2021KSYS009), the National Natural Science Foundation of China (82471386), and the Guangzhou Municipal Science and Technology Project (2024A03J0071).
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest to disclose.
ETHICS STATEMENT
None.




