Pharmacokinetic analysis on compatibility of Atractylodes macrocephala and Paeoniae radix herb pair ameliorates functional constipation model rats using microdialysis with ultra-performance liquid chromatography
Xiaoting Wang, Xiaojun Li, and Rui Zhang have contributed equally to this work.
Abstract
Background
In a previous study, we found that Atractylodes macrocephala and Paeoniae radix (AM-PR) was useful for the alleviation of functional constipation (FC). However, the precise mechanism underlying the compatibility between AM and PR in the treatment of FC remains uncertain. This study aims to analyze the pharmacokinetic differences in the active ingredients in the blood of rat models with FC when applied individually and in combination with AM-PR. It also seeks to compare the changes in the content of the active ingredient when applied individually and in combination with in vitro AM-PR, further in-depth investigation into its material foundation in terms of pharmacokinetics, as well as the composition of the substance.
Methods
Blood microdialysis samples were collected using microdialysis technology. These samples from rats with FC were compared after administration of AM, PR, and AM-PR. The concentration of the main active ingredients was determined using the Ultra Performance Liquid Chromatography-Tunable Ultraviolet (UPLC-TUV) method. The concentration of the main active ingredients of the decoction compatibility before and after combining AM-PR was also determined using the UPLC-TUV method.
Results
Our findings reveal that upon combination, the time to maximum concentration (Tmax) of isochlorogenic acid A (ICA-A) and ataridolide II (ATR-II) Tmax was prolonged, terminal elimination half-life (T1/2) was reduced, and maximum plasma concentrations (Cmax) increased. The Tmax of ataridolide III (ATR-III) remained consistent, whereas its T1/2 and Cmax were significantly reduced. Conversely, for peoniflorin (PAE), Tmax occurred sooner, T1/2 was shortened, and Cmax increased. The Tmax for albiflorin (ALB) remained consistent, whereas T1/2 and Cmax witnessed significant increases. The area under the moment curve (AUMC) (0–t) and AUMC (0–∞) of PAE, ALB, ICA-A, ATR-II experienced an increase after AM-PR administration in rats, attributable to the heightened Cmax. In comparison to individual herb administration, the Tmax of ALB was advanced in combination, the Tmax of PAE remained unchanged, and the Tmax of ICA-A and ART-II was delayed, with an increased area under the concentration–time curve (AUC) (0–t), indicating enhanced Cmax and bioavailability. Furthermore, the dissolution rates of PAE, ICA-A, and ATR-II significantly improved after compatibility.
Conclusions
This study partially clarifies the rationale and compatibility of AM-PR in treating FC and offers a new perspective on the pharmacokinetic interactions of AM-PR in FC treatment.
1 INTRODUCTION
FC also known as habitual constipation or simple constipation, refers to a disease causing primary persistent chronic constipation, which is characterized by dry stool, difficult defecation, reduced defecation, and other symptoms.1, 2 FC is a common disease in adults and children. The cause of FC is unknown in most children, and one-third of children still have problems after puberty. Up to 84% of children with FC have fecal incontinence, whereas more than one-third of children experience major or minor behavioral problems due to constipation.3 FC not only imposes a certain psychological and physical burden on patients and their relatives but also leads to a decline in learning, a reduction in quality of life, a series of negative moods (anxiety and depression), and other problems.4-8 Currently, Western medicine treatments primarily include laxatives to promote bowel movements, gastrointestinal motility drugs to enhance gastrointestinal motility, probiotics to improve the intestinal microenvironment, and dietary interventions.9 However, most of these treatments are symptomatic and do not address the root cause of FC. They often involve long treatment cycles and are difficult to completely cure, and the condition tends to recur after discontinuing the medication. Additionally, laxatives may have several side effects, such as fecal incontinence, bloating, abdominal pain, and nausea.10 In severe cases, the abuse of laxatives by constipation patients leads to laxative-induced constipation and melanosis coli, which makes the treatment of FC even more critical. Traditional Chinese medicine (TCM) typically emphasizes achieving balance between yin and yang to attain overall harmony in treating diseases. Studying single herbs often fails to reflect their true effects within complex formulas, as the multiple ingredients create intricate interactions, making it difficult to draw accurate conclusions. “Herb pairs” as a preliminary form of herb combinations, can complement these studies by providing a more focused approach to understanding the effects and interactions within TCM formulations.11 Yin/yang doctrine is a fundamental concept in TCM, and this could be regarded as its earliest application in the biomedical field. TCM correlates the living system with health and disease using yin/yang. The balance of yin and yang can achieve a healthy state. The characteristics of Chinese herbal medicine are divided based on taste, thermal property, direction, and channel affiliations. By balancing yin and yang, the human body can reach a healthy state by using the corresponding herbs pairs.12, 13
Constipation is intricately linked to intestinal flora and gastrointestinal function. Patients with constipation exhibit significantly higher levels of inflammatory factors compared to the healthy population, leading to a disruption of normal microflora homeostasis in the intestines.14 ALB and PAE are key active components of the total glucosides of paeony in Paeoniae radix, known to regulate colonic motility.15 Research indicates that ALB markedly enhances the reduction of scopolamine-induced acetylcholine (Ach) levels in the striatum of rats, inhibits acetylcholinesterase (AchE) activity in the hippocampus, and reverses the reduction of serotonin (5-HT) and its metabolite 5-HIAA in the prefrontal cortex and hippocampus through intraperitoneal injection.16-18 ICA-A, ART-II and ART-III are primary active components of Atractylodes macrocephala.19 ICA-A exhibits strong anti-inflammatory properties, inhibiting mRNA expression of pro-inflammatory cytokines, blocking phosphorylation of STAT3, p65, and IκBα and suppressing the expression of STAT3 and p65.20 ATR-II aids in regulating gastrointestinal function and enhancing nutrient absorption.21 ATR-III is recognized for its pharmacological properties, including antioxidant, anti-inflammatory, anti-angiogenic, and anti-tumor effects.22-26 Thus, these five components were selected as target components for this study, which subsequently explored their pharmacokinetics.
The “AM-PR herb pair” is a classic drug pair in the clinical upregulation of liver and spleen in TCM, which is used in Danggui Shaoyao San Decoction, Baizhu Shaoyao Powder, and Xiaoyao Powder.27 The AM-PR herb pair has been used to treat a variety of bowel diseases such as ulcerative colitis and other diseases.28-30 In a previous study, we found that AM-PR was useful for alleviating FC.31 However, the precise mechanism underlying the compatibility between AM and PR in the treatment of FC remains uncertain, necessitating further in-depth investigation into its material foundation.
Microdialysis is a kind of in vivo sampling technology based on the principle of dialysis, which can effectively determine the concentration of endogenous or exogenous substances in the extracellular fluid of a variety of tissues (e.g., brain, joint, skin, knee joint, lung), and can be applied to most pharmacokinetic studies. This can effectively reflect the pharmacokinetic changes in drugs in the target site in vivo, improve drug efficacy, and reduce unnecessary side effects.32-36 Microdialysis is unique in that it samples local, unbound concentrations, making it possible to directly link tissue concentrations to pharmacological effects. At the same time, a valuable feature is that repeated sampling with high temporal resolution can be performed in one animal, conserving resources while gaining more information.
This study employed microdialysis technology to examine the pharmacokinetic variances of active constituents in the bloodstream of rats experiencing FC subsequent to intraperitoneal administration of AM, PR, and AM-PR decoctions. This study hopes to clarify the rationale and compatibility of AM-PR in treating FC and offers a new perspective on the pharmacokinetic interactions of AM-PR in FC treatment. The pharmacokinetics behavior results of PAE, ALB, ICA-A, ATR-II, and ATR-III enable a better understanding of the complex mechanisms of absorption, distribution, metabolism, excretion, and safety for future clinical applications. Furthermore, the UPLC technique was employed to assess the concentration of active constituents pre and post in vitro AM and PR pairing, also partly clarifying the rationality and compatibility of the prescriptions containing AM and PR.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
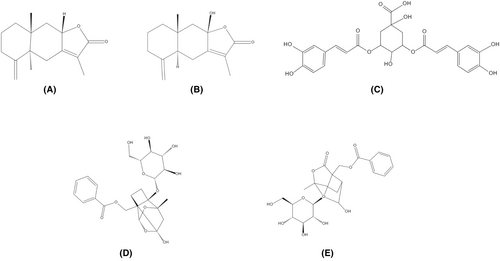
PAE (98 + % pure, lot: PS000825), ATR-II (99 + % pure, lot: PS010511), ATR-III (98.5 + % pure, lot: PS010512), and ICA-A (98.5 + % pure, lot: PS012051) standards were purchased from Chengdu Push Bio-Technology Co., Ltd. (Chengdu, China). ALB (98 + % pure, lot: O31GB166251) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Formic acid (98 + % pure, lot: 64-18-6, analytically pure) was purchased from Hangzhou Changzheng Chemical Reagent Co., Ltd. (Hangzhou, China). Chromatography-grade acetonitrile and methanol were procured from J.T. Baker Chemical Company (New Jersey, USA). Compound diphenoxylate tablets (lot: 2012004) were purchased from Changzhou Kangpu Pharmaceutical Co., Ltd. (Changzhou, China). ATR-II, ATR-III, ICA-A, PAE, and ALB are shown in Figure 1.
2.2 Preparation of TCM
AM (200 g, lot: 220402), PR (200 g, lot: 220701), and AM-PR (each 200 g) were acquired from the Chinese Herbal Pieces Factory of Zhejiang Chinese Medical University (ZCMU) Co., Ltd. (Zhejiang, China) and identified as authentic by Professor Jiaqi Guan (Zhejiang Chinese Medical University, Hangzhou, China). The three drugs were immersed in a tenfold volume of water for a duration of 30 min, followed by a decotion process lasting 1.5 hours. Subsequently, the mixture was filtered, and an additional fivefold volume of water was introduced for 1 hour. After a second filtration, the resulting filtrates were combined on two separate occasions. These three drug groups were concentrated to 0.5 g/mL, 0.5 g/mL, and 1 g/mL, respectively and then stored at 4°C.
2.3 Animals
Forty specific pathogen-free (SPF) healthy female Sprague–Dawley (SD) rats weighing 200 ± 20 g were purchased from ZCMU Laboratory Animal Research Center. They were raised in the Animal Experimental Center of ZCMU. All animal experiments received approval from the Institutional Animal Care and Use Committee at ZCMU and adhered to the applicable animal welfare guidelines (Hangzhou, China, certificate number: IACUC-220815-09). The 40 rats were randomly divided into four groups: normal group, AM group, PR group, and AM-PR group. After a week of acclimation, the FC model was induced using the method by Meng et al.31 The rats were administered diphenoxylate (10 mg/100 g) by oral gavage once daily for 14 consecutive days. Compared with that of the normal group, the stool of model rats had become harder, the rats experienced prolonged defecation, and the particles increased significantly. They were eating less, and they tended to cluster together; their fur was yellowish and dull. The defecation time of the rats was prolonged, and the number of stools increased. These observations indicate the successful modeling of the constipation model.
Following the successful establishment of model, microdialysis was conducted on the animals. Upon completion of the study euthanasia was carried out through exsang uination under pentobarbital anesthesia. The criteria for confirming death in the rats included the absence of respiration, pulse, and heartbeat for a duration exceeding five minutes, in addition to the lack of corneal reflex, pupil dilation, and absence of nerve reflexes. All procedures complied strictly with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health recommendations. The study was conducted in accordance with the ARRIVE guidelines, and the whole experiment was designed to have a human point.
2.4 Liquid chromatography conditions
The liquid chromatography (LC) conditions were as follows: a Welch XB-C18 column (2.1 × 100 mm, 1.8 μm, no.: 00201-11 212); mobile phase: acetonitrile (A)–0.1% formic acid water (B), flow rate 0.3 mL/min, column temperature 30°C, detection wavelength 244 nm; injection volume 3 μL; mobile phase gradient elution procedure: 0–1.5 min (10%A), 1.5–10 min (10%–45%A),10–16 min (45%–65%A), 16–20 min (65%–10%A), 20–25 min (10%A).
2.5 Preparation of standards
Solutions of PAE (4.00 mg/mL), ALB (2.00 mg/mL), ICA-A (1.00 mg/mL), ATR-II (1.00 mg/mL) and ATR-III (1.00 mg/mL) were prepared in methanol as standards. These standards were diluted in methanol to concentrations of 0.40, 0.20, 0.10, 0.10, and 0.10 mg/mL, respectively, and stored at 4°C. The Inblank Ringer's solution (BRS) was formulated with NaCl (1.78 g/100 mL), KCl (55.91 mg/100 mL), MgSO4 (36.11 mg/100 mL), KH2PO4 (13.61 mg/100 mL), NaHCO3 (525.06 mg/100 mL), and CaCl2 (33.294 mg/100 mL). Solutions of PAE, ALB, ICA-A, ATR-II, and ATR-III (each at 1.00 mg/mL) were also prepared in BRS as standards, then diluted to 0.40 mg/mL and stored at 4°C.
2.6 Preparation of test
About 1 mL of the sample was taken under the item “Preparation of TCM,” diluted to 10 mL with methanol, and subjected to ultrasonic extraction for 30 min (100 Hz Kun Shan Ultrasonic Instruments Co., Ltd., Kunshan, China), followed by centrifugation for 10 min (10 000 rpm; centrifuge: HC-3018; Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd., Anhui, China). The supernatant was collected and filtered through a 0.22-μm filter membrane.
2.7 Method validation
2.7.1 Specificity
To determine the specificity of the component of AM-PR, the chromatogram of the tested solution was compared with a standard solution prepared in methanol to investigate whether the tested solution had the same retention time as the standard.
To determine the specificity of the microdialysis experiments, the chromatogram of a standard solution prepared in BRS was observed to investigate its retention time.
2.7.2 Linearity
The linearity of the method was evaluated by separately testing each concentration level of the six standards prepared in methanol and in BRS.
2.7.3 Precision test
Precision was determined by analyzing six replicate concentrations of the mixed standard solution samples within 24 hour.
2.7.4 Repeatability
Six parallel samples were prepared for AM group, PR group, and AM-PR group. Repeatability was determined by analyzing the samples within 24 hour.
2.7.5 Stability
To determine sample stability, the tested solutions were prepared for sequential injections at 0, 2, 4, 6, 8, 10, 12, and 24 hours. Specific peaks over accurate mass ranges were identified, and the area under each standard peak was recorded. The stability was expressed as relative standard deviation (RSD, %).
2.8 Microdialysis experiments
The microdialysis system included a microinjection pump (CMA/400, CMA, Sweden) and a microdialysis sampling system (CMA). Prior to use, the probe was soaked in a heparin sodium solution (1 mg/mL) and perfused at a specific flow rate for 30 min.
Control Ringer's solution (1 μg/mL) was prepared. To investigate the effects of flow rate on probe RL and RR using both reduction and incremental methods, perfusion velocities were investigated at 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 μL/min. The objective of these experiments was to identify the optimal experimental conditions for microdialysis.
Rats were fasted for 12 hour prior to the experiment and had free access to water. After the pentobarbital (40 mg/kg) was administered intraperitoneally, rats were positioned supine and secured using a Self-made retainer. The jugular vein was surgically exposed through blunt dissection, and its distal end was ligated. A small V-shaped incision was made in the vein to facilitate the parallel insertion of blood probes (CMA20, No. T56313, Sweden) toward the heart, ensuring the semipermeable membrane was positioned within the blood vessel and secured with silk sutures, to obtain better rat blood microdialysate samples. The incision was then sutured, and post-surgical BRS was administered to aid in wound healing. After the probe implantation, the AM, PR, and AM-PR groups received the appropriate dosages by duodenal injection (5, 5, and 10 g/kg, respectively). Microdialysate samples were initially collected every 10 min, and after 90 min, every 30 min, with 60 μL per tube, over a continuous period of 8 hour. The probes were initially perfused for 1 hour at a flow rate of 6.0 μL/min, followed by 7 hour at a flow rate of 2.0 μL/min. To maintain anesthesia, sodium pentobarbital was given sublingually every hour throughout the experiment.
2.9 Statistical analysis
SPSS analysis software (SPS S 22.0) was used for the statistical analysis. The precision, repeatability, and stability were expressed as RSD%. All data are expressed by mean ± SD. The pharmacokinetic parameters were obtained by using Drug and Statistics 2.0 (DAS 2.0) software.
3 RESULTS
3.1 General conditions of rats
Compared to the normal group, the stool of model rats had become harder, defecation was delayed, and the number of fecal particles increased significantly. Their appetite diminished and they exhibited a tendency to cluster together; their fur became yellowish and lost its luster. These observations indicate the successful establishment of the FC model.
3.2 Method validation
3.2.1 Specificity
UPLC was employed to analyze the methanol test stock solution and the BRS test solution, with the resulting chromatograms presented in Figures 2 and 3, respectively.
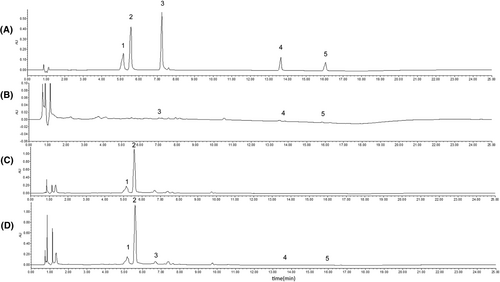
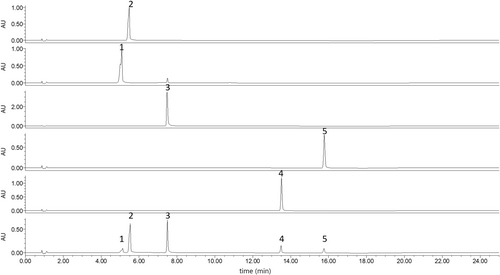
3.2.2 Linearity
The analysis revealed that the linear regression equations for PAE, ALB, ICA-A, ATR-II, and ATR-III in the BRS extraction experiment were as follows: y = 5 709 110.60 x + 268 559.22 (R2 = 0.9993), linear range 4.00 - 0.004 mg·mL−1; y = 5 751 474.77 x + 90 576.48 (R2 = 0.9997), linear range 2.00 - 0.002 mg·mL−1; y = 30 125 201.92 x + 81 757.08 (R2 = 0.9998), linear range 0.50 - 0.001 mg·mL−1; y = 4 589 713.04 x + 44 223.06 (R2 = 0.9996), linear range 1.00 - 0.001 mg·mL−1; y = 6 090 959.34 x + 72 421.37 (R2 = 0.9993), linear range 1.00 0.001 mg·mL−1.
Similarly, the linear regression equations for PAE, ALB, ICA-A, ATR-II, ATR-III in blood microdialysis solution were: y = 10 339x – 4357.9 (R2 = 0.9998), linear range 100 - 0.01 μg·mL−1; y = 9685.8x – 4239.9 (R2 = 0.9998), linear range 100 - 0.01 μg·mL−1; y = 5776.2x – 2743.5 (R2 = 0.9991), linear range 100 - 0.01 μg·mL−1; y = 70.582x + 67.543 (R2 = 0.9991), linear range 100 - 0.05 μg·mL−1; y = 5640.6x – 1158.9 (R2 = 0.9999), linear range 100 - 0.01 μg·mL−1.
3.2.3 Precision test
Precision was established by analyzing six replicate concentrations of mixed standard solution samples, with the chromatographic peak areas recorded and presented in Table 1.
| Ingredients | Peak area | RSD (%) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| PAE | 3 699 071 | 3 736 548 | 3 752 887 | 3 778 213 | 3 751 060 | 3 661 597 | 1.14 |
| ALB | 1 729 673 | 1 741 407 | 1 749 293 | 1 769 090 | 1 753 614 | 1 726 023 | 0.92 |
| ICA-A | 3 605 913 | 3 609 876 | 3 604 955 | 3 588 021 | 3 526 167 | 3 474 371 | 1.56 |
| ATR-II | 587 858 | 593 789 | 593 010 | 606 098 | 598 753 | 595 208 | 1.03 |
| ATR-III | 618 104 | 627 481 | 627 532 | 637 163 | 626 905 | 622 372 | 1.02 |
- Abbreviations: ALB, albiflorin; ATR-II, ataridolide II; ATR-III, ataridolide III; ICA-A, isochlorogenic acid A; PAE, peoniflorin; RSD, relative standard deviation.
3.2.4 Repeatability
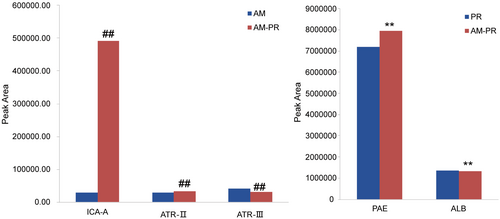
For the AM, PR, and AM-PR groups, six parallel samples were prepared. Repeatability was assessed by analyzing the samples within a 24-hour period and recording the chromatographic peak areas, with the results presented in Table 2. Additionally, Figure 4 illustrates an increase in the dissolution of PAE, ICA-A, and ATR-II.
| Group | Ingredients | Peak area | Mean | RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| AM | ICA-A | 28 984 | 28 990 | 27 544 | 28 995 | 28 081 | 28 126 | 28 453.33 | 2.19 |
| ATR-II | 30 190 | 29 883 | 29 429 | 29 921 | 30 937 | 30 029 | 30 064.83 | 1.65 | |
| ATR-III | 41 771 | 41 798 | 41 585 | 41 349 | 41 097 | 41 562 | 41 527.00 | 0.64 | |
| PR | PAE | 7 179 924 | 7 181 859 | 7 242 153 | 7 049 212 | 7 316 108 | 7 203 888 | 7 195 524.00 | 1.22 |
| ALB | 1 367 912 | 1 374 879 | 1 393 802 | 1 344 036 | 1 369 752 | 1 358 595 | 1 368 162.67 | 1.21 | |
| AM-PR | PAE | 7 989 420 | 7 995 920 | 7 893 944 | 7 912 051 | 7 874 927 | 7 931 174 | 7 932 906.00 | 0.63 |
| ALB | 1 311 470 | 1 332 172 | 1 332 535 | 1 315 353 | 1 328 186 | 1 317 537 | 1 322 875.50 | 0.70 | |
| ICA-A | 494 432 | 494 011 | 494 874 | 491 752 | 488 739 | 490 662 | 492 411.67 | 0.49 | |
| ATR-II | 33 869 | 33 921 | 33 776 | 33 772 | 33 951 | 33 813 | 33 850.33 | 0.22 | |
| ATR-III | 30 749 | 30 816 | 30 163 | 30 375 | 30 598 | 30 662 | 30 560.50 | 0.81 | |
- Abbreviations: ALB, albiflorin; AM, Atractylodes macrocephala; ATR-II, ataridolide II; ATR-III, ataridolide III; ICA-A, isochlorogenic acid A; PAE, peoniflorin; PR, Paeoniae radix; RSD, relative standard deviation.
3.2.5 Stability
To assess sample stability of the samples, solutions of AM, PR and AM-PR were prepared for sequential injections at intervals of 0, 2, 4, 6, 8, 10, 12, and 24 hours. Specific peaks within defined mass ranges were identified, and the area under the curve for each standard's peak was recorded, The results are presented in Table 3.
| Group | Ingredients | Peak area | RSD (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | 14 h | 16 h | 18 h | 20 h | 22 h | 24 h | |||
| AM | ICA-A | 14 347 | 14 469 | 14 638 | 14 429 | 14 595 | 14 448 | 14 511 | 14 458 | 14 472 | 14 752 | 14 613 | 14 072 | 14 103 | 1.34 |
| ATR-II | 10 078 | 10 570 | 10 084 | 10 820 | 10 790 | 10 930 | 10 657 | 10 708 | 11 144 | 10 945 | 10 931 | 10 813 | 10 576 | 2.96 | |
| ATR-III | 18 462 | 18 381 | 19 357 | 18 418 | 18 545 | 18 284 | 18 318 | 18 549 | 18 502 | 18 527 | 19 114 | 18 591 | 18 244 | 1.73 | |
| PR | PAE | 7 614 996 | 7 590 322 | 7 604 095 | 7 658 490 | 7 657 701 | 7 685 245 | 7 644 613 | 7 632 560 | 7 617 126 | 7 564 855 | 7 623 582 | 7 636 554 | 7 632 777 | 0.41 |
| ALB | 1 834 823 | 1 822 969 | 1 822 222 | 1 838 314 | 1 834 236 | 1 840 021 | 1 829 737 | 1 829 820 | 1 820 316 | 1 811 136 | 1 822 173 | 1 825 265 | 1 830 215 | 0.44 | |
| AM-PR | PAE | 7 268 064 | 7 255 486 | 7 339 847 | 7 312 954 | 7 343 399 | 7 359 017 | 7 393 829 | 7 379 940 | 7 430 547 | 7 327 133 | 7 355 378 | 7 366 959 | 7 405 977 | 0.69 |
| ALB | 1 034 113 | 1 033 805 | 1 060 241 | 1 043 693 | 1 051 700 | 1 068 475 | 1 071 833 | 1 071 611 | 1 077 688 | 996 877 | 990 760 | 994 494 | 994 503 | 3.20 | |
| ICA-A | 399 223 | 396 123 | 401 098 | 401 608 | 404 262 | 407 220 | 402 541 | 408 252 | 408 223 | 411 004 | 404 895 | 407 377 | 412 870 | 1.18 | |
| ATR-II | 28 352 | 27 606 | 27 769 | 28 730 | 27 893 | 28 451 | 28 409 | 28 938 | 29 262 | 28 111 | 28 764 | 28 467 | 29 735 | 2.10 | |
| ATR-III | 27 347 | 28 061 | 28 880 | 28 476 | 28 063 | 28 646 | 28 311 | 27 651 | 28 006 | 28 516 | 29 290 | 28 097 | 27 454 | 1.97 | |
3.3 Pharmacokinetic profile
3.3.1 Effect of perfusion velocity on microdialysis
As illustrated in Figure 5, the investigation into the impact of varying perfusion velocities on the recovery rate of the measured components was conducted using the incremental method. The findings indicate a significant inflience of flow rate on the probe's recovery rate, with an inverse ralationship observed as the flow rate increases, the recovery rate of the probe decreased.
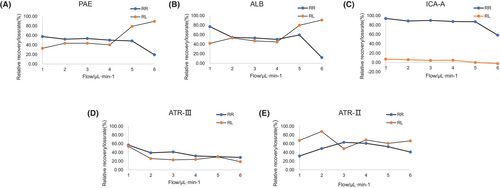
As the results are reported in Figure 5 the effect of the relative loss rate of the measured components under different perfusion velocities was investigated by the decrement method.
3.3.2 The pharmacokinetic differences in the active ingredient
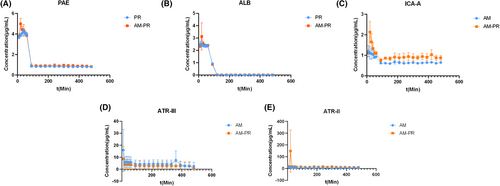
Blood samples, delivered intraduodenally to rats, were analyzed utilizing the UPLC-TUV method. The plasma concentration–time curves for PAE, ALB, ICA-A, ATR-II, and ATR-III in the blood of AM group, PR group, and AM-PR group are illustrated in Figure 6. The DAS2.0 software was employed to analyze the plasma concentration–time profiles of the primary active constituents in FC rats both prior to and following the co-administration of AM and PR drugs. The results are detailed in Table 4.
| Parameter | ALB | PAE | ICA-A | ATR-III | ATR-II | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | AM-PR | PR | AM-PR | AM | AM-PR | AM | AM-PR | AM | AM-PR | |
| Tmax (min) | 20 | 20 | 40 | 20 | 10 | 20 | 10 | 10 | 10 | 20 |
| T1/2 (min) | 2466.30 ± 2553.85 | 6534.22 ± 6138.10 | 7982.58 ± 2242.18 | 5103.95 ± 7651.87 | 1477.32 ± 870.84 | 741.29 ± 271.54 | 5385.12 ± 8428.77 | 407.00 ± 578.24 | 304.06 ± 275.11 | 52.92 ± 44.63 |
| Cmax (μg/mL) | 2.53 ± 0.21 | 3.13 ± 1.10 | 4.23 ± 0.32 | 4.99 ± 0.52 | 1.19 ± 0.28 | 2.13 ± 0.52 | 15.99 ± 17.40 | 8.61 ± 10.19 | 18.87 ± 3.01 | 147.97 ± 178.04 |
| AUC (0 ~ t) (μg/mL*min) | 199.51 ± 6.02 | 206.92 ± 14.60 | 371.43 ± 171.59 | 654.08 ± 46.86 | 326.56 ± 13.33 | 456.70 ± 33.61 | 2242.63 ± 1679.50 | 1425.51 ± 1674.42 | 3062.09 ± 1472.98 | 7213.09 ± 992.90 |
| AUC (0 ~ ∞) (μg/mL*min) | 232.87 ± 33.29 | 288.97 ± 86.08 | 766.91 ± 219.86 | 6629.26 ± 8842.79 | 1667.04 ± 760.47 | 1365.23 ± 206.84 | 9296.36 ± 5177.11 | 1829.95 ± 1409.31 | 6163.72 ± 4097.11 | 7903.17 ± 1833.39 |
| AUMC (0 ~ t) | 9923.13 ± 154.20 | 10 070.48 ± 432.66 | 103 073.00 ± 3095.88 | 111 043.63 ± 122 82.44 | 73 647.67 ± 937.39 | 105 543.97 ± 14 204.91 | 481 039.62 ± 362 532.62 | 310 937.64 ± 367 758.49 | 667 400.35 ± 419 161.53 | 1 357 763.73 ± 553 987.56 |
| AUMC (0 ~ ∞) | 230 661.13 ± 338 928.52 | 1 275 330.55 ± 1 539 442.62 | 117 045 772.35 ± 6 540 6072.64 | 112 102 138.35 ± 191 463 832.00 | 4 313 359.89 ± 3 322 734.83 | 1 587 019.93 ± 722 701.52 | 94 335 771.48 ± 146 632 457.81 | 1 081 424.67 ± 1 060 516.26 | 3 821 167.36 ± 2 905 777.61 | 2 029 611.29 ± 1 456 309.54 |
| MRT (0 ~ t) (min2) | 49.76 ± 1.29 | 48.73 ± 1.42 | 167.41 ± 3.82 | 169.51 ± 7.89 | 225.74 ± 8.20 | 230.43 ± 15.53 | 213.52 ± 16.84 | 216.10 ± 3.45 | 208.30 ± 37.62 | 187.01 ± 65.91 |
| MRT (0 ~ ∞) (min2) | 875.34 ± 1234.40 | 3632.81 ± 3930.98 | 11 265.98 ± 3242.73 | 7192.39 ± 10 928.29 | 2207.49 ± 1256.94 | 1128.47 ± 388.61 | 7815.10 ± 12 056.94 | 707.08 ± 725.02 | 567.76 ± 396.99 | 242.19 ± 121.28 |
- Note: Experimental data are mean ± SD.
Table 4 indicates that upon combination, the time to maximum concentration (Tmax) of ICA-A and Tmax of ATR-II were prolonged, terminal elimination half-life (T1/2) was reduced, and maximum plasma concentrations (Cmax) increased. The Tmax of ATR-III remained consistent, whereas its T1/2 and Cmax were significantly reduced. Conversely, for PAE, Tmax occurred sooner, T1/2 was shortened, and Cmax increased. The Tmax for ALB remained consistent, whereas T1/2 and Cmax saw significant increases. The MRT (0–t) for each group shows a minimal change. The area under the moment curve AUMC (0–t) and AUMC (0–∞) for PAE, ALB, ICA-A, and ATR-II were elevated after AM-PR administration in rats. Compared to individual herbs, the Tmax of ALB increased post-matching, the Tmax of PAE remained unchanged, the Tmax of ICA-A and ART-II was delayed, and the AUC (0–t) increased, indicating an increase in both Cmax and bioavailability after matching.
4 DISCUSSION
FC has been shown to cause psychological burden and decrease quality of life due to its long course and recurrent symptoms that are difficult to treat.37, 38 According to evidence-based medicine, Chinese medicine demonstrates specific advantages in the management of constipation.39, 40 There are various chemical components present in AM, including volatile oils, polysaccharides, lactones, sesquiterpenoids, and glycosides41, 42 ,of which sesquiterpenoids are a major active constituent of AM43 At present, there are more than 60 kinds of sesquiterpenoids isolated from AM, mainly ATR-II, ATR-III, and so on.44 Intestinal function and gut microflora are regulated by AM, and it can improve intestinal flora disorder.41, 45 In addition, as one of the components of AM, ICA-A alleviates ulcerative colitis by inhibiting the STAT3/NF-кB signaling pathway.20
The main chemical components of PR include triterpenes, monoterpenes, phenolic acids, fatty acids, and other compounds.46 The total glucosides of paeony are the main active ingredients of PR, mainly including monoterpene glycosides such as PAE and ALB, which are the basis of the pharmacodynamic substance of PR.15 Currently, PR is widely used in traditional treatments for stomach pain as well as intestinal problems.46
In this study, we further investigated the substance basis and mechanism of AM-PR in the treatment of FC and found that mixed extraction of AM and PR could promote the dissolution of PAE, ICA-A, and ATR-II. Yanhong Wang et al.47 found that the contents of ATR-II and ATR-III increased significantly after compatibility, whereas the contents of PAE and ALB decreased significantly. The results were somewhat different from those in this paper, which may be due to the fact that the former used bran after stir-frying AM and PR, and the raw AM and PR used in this paper. In addition, the producing area is one of the most important factors that could affect the content of TCM. In this study, the content of the active ingredients was compared before and after compatibility by controlling the variable method, which suggested that AM and PR may play a synergistic role in the treatment of FC. While promoting the compatibility of the two herbs, the mixed extraction of the two herbs promotes and inhibits the dissolution of chemical components through the interaction of different herbal chemicals. The above may preliminarily explain the synergistic mechanism of the compatibility of TCM.
We found that the concentration of each component to be measured showed a trend of first increasing and then decreasing, on the drug time curve, which indicated the process of absorption, distribution, and metabolic elimination of drug components in the body and conformed to the typical oral metabolism characteristics of drugs. The components of AM and PR are absorbed quickly in the body.
Tmax reflects the speed at which the drug enters the body, T1/2 directly reflects the rate of drug elimination from the body. Cmax reflects the important indicators of the rate and extent of drug absorption in the body.48, 49 Compatibility may improve the therapeutic effect of FC by changing the rate at which the drug enters the body, the rate at which it is excreted from the body, and the degree to which the drug is absorbed in the body. The MRT (0–t) of each group has not changed much. The AUMC (0–t) and AUMC (0–∞) of PAE, ALB, ICA-A, ATR-II were elevated after administration of AM-PR in rats, which is likely caused by elevated Cmax. Generally, these monoterpene glycosides could be absorbed and their clearance in vivo is fast.50 Compared with individual herbs, the Tmax of ALB was advanced after matching, the Tmax of PAE remained unchanged, the Tmax of ICA-A and ART-II was delayed, and AUC (0–t) increased, indicating that Cmax and bioavailability increased after matching.
The components in the blood are complicated, and the endogenous substances greatly interfere. The blood concentration method was mostly adopted in traditional blood sample analysis, which requires tedious operations such as stratification, centrifugation, extraction, and filtration before injection analysis, which greatly affects the accuracy and efficiency of drug research. Based on the principle of semi-permeable membrane, the microdialysis technology ensures that collected samples do not contain macromolecular substances such as proteins, which avoids the interference of macromolecular substances, greatly reduces the loss of body fluids, and ensures the well-being of the animal. In this study, we found that probe recovery is greatly affected by flow rate, and the larger the flow rate, the lower the probe recovery. By combining microdialysis with pharmacokinetics, sampling times should be intensive, and probe recovery is higher at smaller flow rates, but the amount of sample collected is reduced. Therefore, when applying microdialysis technology in vivo research, it should be ensured that the sample size is sufficient to reach the detection amount of the instrument, and researchers should try to choose high-flow perfusion as much as possible. In the future, we envision microdialysis being able to measure the kinetics of biochemical reactions near microdialysis probes, enabling deeper insights into tissue function, disease, and ways to monitor treatments to provide optimal care. Such information is clearly more valuable than simply focused knowledge.
In summary, this study explored the potential material basis of the AM-PR herb pair for the amelioration of FC and found that their combined use can promote the dissolution of PAE, ICA-A, ATR-II, and also improve the bioavailability of PAE, ALB, ICA-A, and ART-II. However, further exploration of the mechanism is still required.
5 CONCLUSION
In this study, the dissolution rates of PAE, ICA-A, and ATR-II were found to significantly increase following the compatibility of AM and PR. By examining the effect of AM and PR compatibility extraction on active ingredients, a combined microdialysis and UPLC method was developed and validated. This method was used to study the pharmacokinetics of PAE, ALB, ICA-A, ATR-II, and ATR-III in the blood of rats with FC after the administration of AM, PR, and AM-PR. Our results indicate an increase in the bioavailability of PAE, ALB, ICA-A, and ATR-II post-compatibility. It provides a new perspective to elucidate the pharmacokinetic interactions of AM-PR in the treatment of FC. The enhanced efficacy of AM-PR may be attributed to the improved bioavailability of these compounds and the increased dissolution rates of PAE, ALB, ICA-A, and ATR-II. Consequently, this research offers valuable insights into the in vivo metabolic processes of FC influenced by the AM-PR herb pair. It also sheds light on the rationality and compatibility of prescriptions containing AM and PR and underscores the potential therapeutic benefits of the AM-PR herb pair in treating FC. It offers a new approach for the future treatment of clinical FC.
AUTHOR CONTRIBUTIONS
Xiaoting Wang: Conceptualization; data curation; investigation; methodology; writing – original draft. Xiaojun Li: Formal analysis; investigation; validation; visualization. Rui Zhang: Project administration; supervision; writing – review and editing. Yin Hong: Formal analysis; funding acquisition; software; writing – review and editing. Jiaqi Guan: Conceptualization; funding acquisition; methodology; resources; supervision; writing – review and editing.
ACKNOWLEDGMENTS
We appreciate the experimental support from the Public Platform of Medical Research Center and the Public Platform of Pharmaceutical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
FUNDING INFORMATION
This study was supported by Natural Science Foundation of Zhejiang Province (no.: Y19H280022).
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no competing interests.
ETHICS STATEMENT
All animals were humanely cared in Zhejiang Chinese Medical University (IACUC-220815-09, Zhejiang Province, P.R. China). The study was guided and approved by Animal Ethical and Welfare Committee of ZCMU.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.




