The N-terminal domain of gasdermin D induces liver fibrosis by reprogrammed lipid metabolism
Abstract
Background
The emerging incidence of pathogenic liver conditions is turning into a major concern for global health. Induction of pyroptosis in hepatocytes instigates cellular disintegration, which in turn liberates substantial quantities of pro-inflammatory intracellular substances, thereby accelerating the advancement of liver fibrosis. Consequently, directing therapeutic efforts towards inhibiting pyroptosis could potentially serve as an innovative approach in managing inflammation related chronic hepatic disorders.
Methods
GSDMD-NTki/wt mice and Alb-creki/wt mice were generated using CRISPR/Cas9 technology. After crossing the two strains together, we induced conditional cell death by doxycycline to construct a mouse model of liver fibrosis. We analyzed differentially expressed genes by RNA sequencing and explored their biological functions. The efficacy of obeticholic acid (OCA) in the treatment of liver fibrosis was assessed.
Results
Doxycycline-treated GSDMD-NTki/wt × Alb-creki/wt mice showed severe liver damage, vacuolation of hepatocytes, increased collagen fibers, and accumulation of lipid droplets. The expression of liver fibrosis related genes was greatly increased in the doxycycline-treated mouse liver compared with untreated mouse liver. RNA-sequencing showed that upregulated differentially expressed genes were involved in inflammatory responses, cell activation, and metabolic processes. Treatment with OCA alleviated the liver fibrosis, with reduced ALT and AST levels seen in the GSDMD-NTki/wt × Alb-creki/wt mice.
Conclusions
We successfully constructed a novel mouse model for liver fibrosis. This GSDMD-NT-induced fibrosis may be mediated by abnormal lipid metabolism. Our results demonstrated that we successfully constructed a mouse model of liver fibrosis, and GSDMD-NT induced fibrosis by mediating lipid metabolism.
1 INTRODUCTION
Chronic liver disease (CLD) is a severe global health concern and is increasingly garnering widespread attention. Millions of individuals each year are diagnosed with CLD, imposing a substantial burden on the global healthcare system.1 The etiology of chronic liver disease is multifaceted and includes factors such as chronic viral infections, alcoholic steatohepatitis (ASH), non-alcoholic steatohepatitis (NASH), and chronic liver diseases stemming from autoimmune aberrations and genetic predispositions. The interplay of these factors gives rise to the intricate pathogenesis of CLD. Liver fibrosis is a pathological hallmark observed in a spectrum of chronic liver diseases. It has the potential to progress to severe stages such as cirrhosis and hepatocellular carcinoma if not effectively managed. A pivotal event in the fibrogenic process is the activation of hepatic stellate cells (HSCs), which is intricately linked with lipid metabolism.2 Perturbations in fatty acid metabolism are common denominators in various fibrotic disorders. Dysregulation leading to excessive lipid accumulation or impaired fatty acid oxidation can result in lipotoxicity, a key contributor to fibrosis. Genetic modifications or pharmacological interventions targeting fatty acid metabolic pathways hold promise for mitigating fibrosis.3 In the context of metabolic syndrome, there is limited evidence directly linking changes in metabolites such as glucose and free fatty acids to HSC transdifferentiation. However, these metabolic alterations may lead to a decrease in immunomodulatory and hepatoprotective molecules, including lipoxins, resolvins, and interleukin-22 (IL-22).4 Furthermore, lipotoxicity-induced cellular stress and hepatocyte apoptosis are key drivers of non-alcoholic fatty liver disease (NAFLD) and can facilitate its progression to NASH.5, 6 The leading causes of liver disease worldwide are NAFLD and NASH.7, 8 Hepatic steatosis, inflammation, hepatocellular injury, and varying degrees of fibrosis characterize NASH, a progressive form of NAFLD. Furthermore, NASH is associated with accelerated disease progression and an increased risk of hepatocellular carcinoma.9 Although no drugs have been approved for NASH, continuous progress in elucidating the pathogenesis and identifying therapeutic targets is being made.10 For example, obeticholic acid (OCA), chemically known as 6α-ethyl-chenodeoxycholic acid, has shown efficacy in improving various liver diseases, including fibrosis.11 The development of OCA was predicated on the identification of chenodeoxycholic acid, a natural bile acid, as the most potent physiological ligand for the farnesoid X receptor (FXR).12 FXR plays a significant role in various physiological and pathological processes. A series of alkylated bile acid analogues were synthesized and evaluated, with 6α-ethyl-chenodeoxycholic acid demonstrating the highest potency as an FXR agonist. Recently, OCA has undergone clinical trials for the treatment of NASH and is currently considered the most promising candidate drug in this regard.13
Inflammatory caspases are mediators of inflammation and play important roles in innate immune defenses.14 A growing number of studies suggests that unusual or excessive stimulation of inflammatory caspases is linked to the development of diseases such as alcoholic liver disease (ALD), NASH, and hepatitis C virus infection.15-17 Gasdermin D (GSDMD) is part of the gasdermin family, which consists of two fragments of 31 kDa N-terminal (GSDMD-NT) and 22 kDa C-terminal (GSDMD-CT) linked by a peptide junction.18 The junction cleaves in response to exogenous stimuli or inflammation-induced activation, separating the GSDMD-NT from its autoinhibitory domain GSDMD-CT. The GSDMD-N then forms a transmembrane pore, which facilitates the release of cytokines like interleukin-1β (IL-1β) and interleukin-6 (IL-6), ultimately leading to pyroptosis and subsequent downstream effects, such as liver fibrosis.19-21 While cell death mediated by GSDMD and the downstream inflammation serve as crucial self-defense mechanisms in response to exogenous stimuli and infections, aberrant activation of GSDMD can lead to severe inflammatory cascades. These abnormal events include disruptions in ion homeostasis, impairment of organelle function, cell membrane rupture, and sustained release of inflammatory cytokines. These aberrant responses may contribute to the development of various diseases, including sepsis, neurodegenerative disorders, NASH, and malignancies.22 Research on GSDMD may thus aid in understanding inflammation regulation and disease pathogenesis and lead to the development of therapeutic strategies. While a pivotal role of pyroptosis in immunity and disease has been demonstrated,23, 24 the mechanism of GSDMD-driven pyroptosis in liver fibrosis has not been elucidated.
In this research, we developed a mouse model with inducible liver-specific GSDMD-NT expression to investigate the significance of GSDMD-NT in liver fibrosis. We examined the role of GSDMD-NT in the pathogenesis of fibrosis. Furthermore, we explored the impact of GSDMD-NT in NASH and demonstrated a positive correlation between GSDMD-NT and NASH.
2 METHODS
2.1 Patient samples
Two liver biopsy samples with high grade fibrosis and one non-liver fibrosis specimen were collected in the Fatty Liver Disease Center of integrated Chinese and Western medicine, Affiliated Hospital of Nanjing University of Chinese Medicine from January 2022 to June 2022. Medical ethics committee approval of specimen collection was obtained from Nanjing University of Chinese Medicine's Affiliated Hospital (Nanjing, China, approval no.: 2022NL-014002), and written informed consent was obtained from the patients. The samples were treated and analyzed as in our previous report.25
2.2 Mouse models
All mice used in this study were housed in a pathogen-free animal facility at a temperature of 23 ± 2°C, a relative humidity of 50 ± 10%, and a 12-h light/dark cycle. Mice were provided with regular chow or a custom diet and had free access to autoclaved water.
The H11-pTRE-GSDMD-CAG-lsl-rTTA-IRES-ZsGreen (GSDMD-NTki/wt) mouse (C57BL/6J) was created at GemPharmatech Co. Ltd. We used gene editing technology to insert the pTRE-GSDMD-CAG-lsl-rTTA-IRES-ZsGreen gene fragment into the mouse H11 site in the mouse model. Briefly, Cas9donor and sgRNA were co-injected into the fertilized embryos of mice to obtain F0 generation mice. F0 generation mice were verified by PCR, sequencing and southern blot and then mated with C57BL/6J mice to obtain F1 generation mice.
GSDMD-NTki/wt mice were mated with Alb-Cre mice (C57BL/6 strain) to obtain GSDMD-NTki/wt × Alb-creki/wt mice. In some experiments, GSDMD-NTki/wt mice and GSDMD-NTki/wt × Alb-creki/wt mice were given free access to drinking water containing doxycycline hydrochloride (dox; Sangon Biotech Co., Shanghai, China), which was changed twice a week. Dox (15 μg/mL) was suspended in the freely available drinking water for 2, 4 or 6 weeks.
In other experiments, mice were randomly divided into control and OCA (30 mg/kg/day, intragastric, i.g.) groups. OCA was suspended in 0.5% sodium carboxymethyl cellulose (Aladdin, Shanghai, China) and orally administered once a day from the third week. During the administration period, mice also had free access to drinking water containing dox.
In other experiments, GSDMD-NTki/wt × Alb-creki/wt mice were fed a high-fat and high-sugar diet (HFHS) or a control diet (Research Diets, USA) for 11 weeks to induce steatosis and freely drank dox-containing water from the 8th week; the concentration of dox was gradually increased.
All experimental procedures involving animals in this study were conducted in accordance with protocols that were thoroughly reviewed and given approval by the Institutional Animal Care and Use Committee (IACUC) at GemPharmatech Co. Ltd. (approval no.: CDAP20211018-1#).
2.3 Isolation and culture of primary hepatocytes
As previously described, primary hepatocytes were isolated from mice using a two-step perfusion method.24 The isolated hepatocyte suspension was seeded on collagen-coated dishes at a density of 5 × 104/mL and used for subsequent experiments.
2.4 CCK-8 assay
The CCK-8 kit (Cell Counting Kit-8; Dojindo, Japan) was used to detect cell viability, following the guidelines provided by the manufacturer. Primary hepatocytes were incubated with different concentrations of dox (2, 5, 10, 20, 100, and 500 ng/mL; Sangon Biotech Co., Shanghai, China) for 48 h.
2.5 LDH assay
LDH release in the supernatant was quantified using the LDH Cytotoxicity Assay Kit (Shanghai BeoTeam Biotechnology Co., Ltd) according to the manufacturer's instructions.
2.6 Western blot
Total proteins in liver tissue or primary hepatocytes were extracted with RIPA lysis buffer (Beyotime Co., Shanghai, China). After quantification of protein concentrations using the bicinchoninic acid assay, proteins were electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide electrophoresis gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% skimmed milk, membranes were incubated with corresponding primary antibodies (Table 1). Detection was achieved using an UltraSignal Ultrasensitive Chemiluminescence Detection Kit after incubation with secondary antibody (4A Biotech Co., Beijing, China).
| Name | Application | Host | Dilution ratio | Company |
|---|---|---|---|---|
| Primary antibodies | ||||
| GSDMD-FL | WB | Rabbit | 1:1000 | Abcam |
| GSDMD-NT | WB | Rabbit | 1:1000 | Abcam |
| α-SMA | WB | Rabbit | 1:500 | Servicebio |
| FASN | WB | Rabbit | 1:5000 | Proteintech |
| Scd1 | WB | Rabbit | 1:2000 | Proteintech |
| Acaca | WB | Rabbit | 1:10 000 | Proteintech |
| PPARg | WB | Rabbit | 1:1000 | Proteintech |
| Tubulin-α | WB | Rabbit | 1:3000 | Proteintech |
| GAPDH | WB | Rabbit | 1:50 000 | Proteintech |
| CD68 | IHC | Rabbit | 1:250 | Servicebio |
| GSDMD | IHC | Rabbit | 1:800 | Proteintech |
| Secondary antibodies | ||||
| Goat anti-rabbit IgG (H + L) | IHC | Goat | 1:400 | Biosharp |
| Anti-rabbit IgG HRP | WB | Goat | 1:10 000 | YEASEN |
2.7 ELISA analysis
Evaluation of IL-1β, transforming growth factor β1 (TGF-β1), and chemoattractant protein-1 (MCP-1) in serum and liver homogenates was performed using enzyme-linked immunosorbent assays (ELISA) (Elabscience, Wuhan, China) in accordance with the manufacturer's instructions.
2.8 RNA-sequencing and gene expression analysis
RNA from liver was extracted with GSDMD-NTki/wt and GSDMD-NTki/wt × Alb-creki/wt mice. The purity of the RNA was determined using a NanoPhotometer® (Implen, CA, USA), and RNA integrity and concentration were determined using the Agilent Bioanalyzer 2100 system with an RNA Nano 6000 Assay kit (Agilent Technologies, CA, USA). We generated strand-specific libraries after depleting rRNA using the Ribo-Zero™ Gold Kit (Illumina, San Diego, CA, USA). The libraries were sequenced on an Illumina NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA) using paired-end 150 bp sequencing reads (PE150) at Annoroad Gene Technology Co. Ltd. (Beijing, China). The raw and processed data were uploaded to NCBI Gene Expression Omnibus (GEO) under accession code GSE252989.
After removing the adaptor and low-quality, empty, ribosomal (r)RNA reads from raw sequencing data. The processed high-quality reads were mapped to the mouse reference genome (GRCm39) by HISAT2 2.1.0.26 Mapped reads were assembled and quantified by StringTie v1.3.3,27 and the expression levels of each mRNA were defined by their FPKM values. mRNAs with FPKM greaters than 0.5 in at least two biological replicates were retained. Log2-transformed values of FPKM+1 were used for further analysis. Differentially expressed genes (DEGs) were identified using edgeRbase on gene count.28 Significant DEGs were defined as false discovery rate <0.05 and absolute fold change (FC) > 1.5. Gene ontology (GO) terms enrichment was performed with the clusterProfiler 4.0.29 GO terms with an adjusted p value <0.05 were considered significant.
2.9 RNA extraction and qRT-PCR
Trizol reagent (Invitrogen, CA, USA) was used to extract RNA from primary hepatocytes and frozen liver tissues. As directed by the manufacturer, complementary DNA synthesis was conducted using a PrimeScript reverse transcriptase kit (Takara, Osaka, Japan). Table 2 lists the primer sequences used for the real-time PCR using SYBR Green qPCR Master Mix (Vazyme, Nanjing, China).
| Gene | Sequence | |
|---|---|---|
| Fasn | Forward | GGAGGTGGTGATAGCCGGTAT |
| Reverse | TGGGTAATCCATAGAGCCCAG | |
| Scd1 | Forward | AGTCAGGAGGGCAGGTTTC |
| Reverse | GTAAGAACTGGAGATCTCTTGGA | |
| Acaca | Forward | ATGGGCGGAATGGTCTCTTTC |
| Reverse | TGGGGACCTTGTCTTCATCAT | |
| Acss2 | Forward | AAACACGCTCAGGGAAAATCA |
| Reverse | ACCGTAGATGTATCCCCCAGG | |
| Gpam | Forward | ACAGTTGGCACAATAGACGTTT |
| Reverse | CCTTCCATTTCAGTGTTGCAGA | |
| Dgat1 | Forward | GCCTTACTGGTTGAGTCTATCAC |
| Reverse | GCACCACAGGTTGACATCC | |
| PPARα | Forward | AACATCGAGTGTCGAATATGTGG |
| Reverse | CCGAATAGTTCGCCGAAAGAA | |
| PPARg | Forward | TCGCTGATGCACTGCCTATG |
| Reverse | GAGAGGTCCACAGAGCTGATT | |
| Col1a1 | Forward | TAGGCCATTGTGTATGCAGC |
| Reverse | ACATGTTCAGCTTTGTGGACC | |
| Col1a2 | Forward | CCCAGAGTGGAACAGCGATTA |
| Reverse | ATGAGTTCTTCGCTGGGGTG | |
| Col3a1 | Forward | CCTGGCTCAAATGGCTCAC |
| Reverse | GACCTCGTGTTCCGGGTAT | |
| Col4a2 | Forward | GGACCCAAGGGACAACCAG |
| Reverse | CCCAACAAGTGTGATGTCAGAT | |
| Acta2 | Forward | GCACCCAGCATGAAGATCAAG |
| Reverse | TCTGCTGGAAGGTAGACAGCGAAG | |
| Timp-2 | Forward | TCAGAGCCAAAGCAGTGAGC |
| Reverse | GCCGTGTAGATAAACTCGATGTC | |
| Gapdh | Forward | TTGATGGCAACAATCTCCAC |
| Reverse | CGTCCCGTAGACAAAATGGT |
2.10 Histological analysis
Fixed tissues were washed, embedded in paraffin, and sectioned. Liver sections were stained with Hematoxylin and Eosin (H&E) for histopathological evaluation. Assessment of liver fibrosis was performed by Sirius Red staining and Masson's trichrome staining. The fat in the liver tissue was detected by Oil Red O fat staining. Immunohistochemical staining was performed for GSDMD, α-SMA, and CD68 (Table 1). Histological analysis was performed.
2.11 Biochemical analysis
An automatic blood biochemical analyzer (Beckman Counter LX20, USA) was used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), and total cholesterol (TC) levels in the serum.
2.12 Statistical analysis
The mean ± SEM are used for the statistical analysis of quantitative results. And statistical analysis was performed using GraphPad Prism 9 software. Two groups were compared by two-tailed Student's t-test. One-way analysis of variance with Tukey's multiple comparison test was used to compare groups. Experiments were independently repeated more than three times and p values <0.05 were considered statistically significant.
3 RESULTS
3.1 GSDMD-N induces pyroptosis and causes liver fibrosis
We assessed full-length Gasdermin D (GSDMD-FL) expression in liver biopsies obtained from both NASH patients and healthy subjects as previously described.30 Hepatic GSDMD-FL expression was elevated in NASH patients compared with controls (Figure S1A), suggesting significant hepatic GSDMD-FL production in NASH patients. We also evaluated GSDMD-FL expression in liver biopsies obtained from both fibrotic patients and healthy subjects. Hepatic GSDMD-FL expression was elevated in fibrotic patients, as indicated by GSDMD staining (Figure S1B).
To examine the role of GSDMD-NT-mediated pyroptosis in liver fibrosis, we inserted GSDMD-NT into the mouse genome using the Tet-on inducible expression system and the Cre-loxp recombination system using CRISPR/Cas9. A transgenic mouse model (GSDMD-NTki/wt × Alb-creki/wt) with dox-inducible liver-specific expression of GSDMD-NT was obtained (Figure 1A). We first optimized the concentrations of dox. We found that when the concentration of dox exceeded 15 μg/mL, the survival of mice was poor, we therefore selected 15 μg/mL for subsequent experiments. Induction of GSDMD-NTki/wt × Alb-creki/wt mice with 15 μg/mL dox resulted in noticeable liver damage (Figure 1B). We assessed the expression of GSDMD-FL and GSDMD-NT proteins in the liver tissues of GSDMD-NTki/wt × Alb-creki/wt mice. The liver tissues in mice induced by dox exhibited an increase in GSDMD-NT expression and a decrease in GSDMD-FL expression compared with levels in mice not treated with dox (Figure 1C).
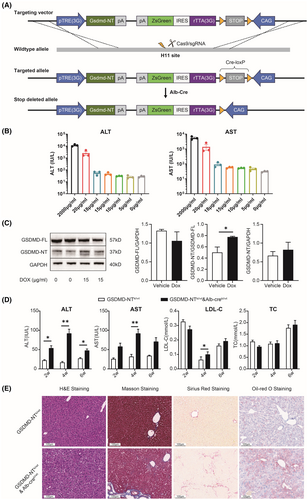
To examine the role of GSDMD-NT in the development of liver fibrosis, GSDMD-NTki/wt × Alb-creki/wt mice were treated with dox for 6 weeks to induce a pathological condition. Compared with mice not treated with dox, dox-induced mice showed increased ALT and AST levels in serum (p < 0.05), but no significant effects in LDL-C and TC were observed (Figure 1D). H&E and Oil-red O staining showed hepatocyte vacuolation and hepatic steatosis in GSDMD-NTki/wt × Alb-creki/wt mice compared with controls. Examination of liver sections using Masson and Sirius Red staining methods demonstrated a notable augmentation in the fibrotic area (Figure 1E). These findings indicated that GSDMD-NT-mediated pyroptosis induced liver fibrosis.
We next isolated primary hepatocytes from GSDMD-NTki/wt and GSDMD-NTki/wt × Alb-creki/wt mice. We first examined the cytotoxicity of dox on primary hepatocytes using CCK-8 assays. Dox had no significant impact on the cell viability of primary hepatocytes from GSDMD-NTki/wt mice; however, it significantly affected the cell viability of cells obtained from GSDMD-NTki/wt × Alb-creki/wt mice (Figure S2A). Furthermore, there was a notable increase in LDH release in the supernatants of primary hepatocytes from GSDMD-NTki/wt × Alb-creki/wt mice treated with dox, which suggests that primary hepatocytes underwent cell death as a result of compromised membrane integrity (Figure S2B).
To examine whether dox-induced cell death was mediated by GSDMD-NT, we conducted western blotting to assess the expression levels of GSDMD-FL and GSDMD-NT proteins. Both levels were significantly increased in primary hepatocytes from GSDMD-NTki/wt × Alb-creki/wt mice after treatment with dox (Figure S2C). These findings collectively indicate the induction of GSDMD-NT-mediated pyroptosis in hepatocytes from GSDMD-NTki/wt × Alb-creki/wt mice by dox.
3.2 GSDMD-NT induces the dysregulation of lipid metabolism-related genes
In order to explore how GSDMD-NT induces fibrosis, we performed RNA-sequencing on liver samples from GSDMD-NTki/wt and dox-treated GSDMD-NTki/wt × Alb-creki/wt mice. A total 697 differentially expressed genes were identified, of which 379 were upregulated and 318 were downregulated (FDR <0.05 and |log2FC|>1, Figure 2A, Table S1). The gene expression patterns were completely different between the two groups (Figure 2B). Genes with differential expression were enriched with GO terms. In the GSDMD-NTki/wt × Alb-creki/wt dox-treated group, the upregulated genes exhibited enrichment in biological processes such as metabolic processes (unsaturated fatty acid metabolic process and icosanoid metabolic process), immune responses (regulation of immune effector process and T cell–mediated cytotoxicity) and cells (regulation of mononuclear cell proliferation) (Figure 2C).
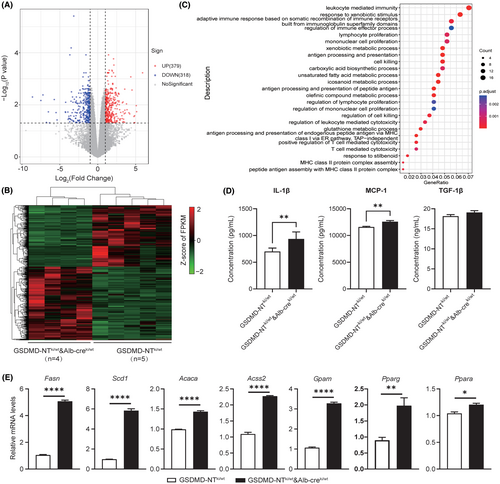
We next assessed the hepatic levels of specific cytokines, including monocyte chemoattractant protein-1 (MCP-1), IL-1β, and transforming growth factor β1 (TGF-β1). The levels of IL-1B and MCP-1 cytokines were significantly increased in the GSDMD-NTki/wt × Alb-creki/wt group compared with controls, but there were no significant differences in TGF-β1 (Figure 2D).
We next evaluated the expression of genes involved in the regulation of hepatic lipid metabolism. Compared with untreated GSDMD-NTki/wt × Alb-creki/wt mice, dox-induced mice exhibited elevated expression of mRNAs related to triglyceride synthesis, fatty acid synthesis, and fatty acid oxidation (Figure 2E). After verification at the gene level, we conducted verification at the protein level. The results showed that, compared with untreated GSDMD-NTki/wt × Alb-creki/wt mice, FASN, Scd1, Acaca, and PPARg proteins in the liver tissue of dox-induced mice were significantly up-regulated, which was consistent with the expression at the gene level (Figure S3A). These results suggested that GSDMD may influence liver fibrosis by regulating lipid metabolism-related genes.
3.3 OCA alleviates GSDMD-NT-driven liver fibrosis
Given the potential therapeutic role of OCA in liver fibrosis, we asked whether OCA has a therapeutic effect in mouse model. We assessed changes in the levels of blood biochemistry-related indicators and performed H&E staining of liver sections. GSDMD-NTki/wt × Alb-creki/wt mice treated with OCA showed a significant reduction in the levels of ALT, AST, LDL-C, and TG, indicative of OCA's ability to restore liver function (Figure 3A). H&E staining showed reduced vacuolation in hepatocytes from OCA-treated mice compared with controls. Meanwhile, OCA treatment also reduced the area of liver parenchyma occupied by fibrotic tissue in Masson and Sirius Red stained liver sections. Oil red O staining results showed that lipid accumulation was significantly reduced after OCA treatment (Figure 3B).

Active HSCs play a crucial role in liver fibrosis progression. We therefore investigated whether OCA influences this activation process. The results showed significantly reduced macrophage infiltration and also a decrease in the number of α-SMA-positive cells after OCA treatment (Figure S4A). Additionally, OCA treatment led to a decrease in the hepatic production of pro-inflammatory cytokines (Figure S4B). These findings collectively suggested that OCA has the potential to mitigate inflammation and HSC activation. In addition, multiple fibrosis markers were significantly reduced by OCA administration as shown by q-PCR (Figure 3C). We further found that OCA significantly reduced the mRNA and protein levels of Scd1, Acaca, Dgat1, Pparg, and FASN, which are involved in lipid metabolism (Figure 3D and Figure S3B). Our results collectively suggested that OCA treatment may relieve liver fibrosis through inhibition of macrophage infiltration, suppression of HSC activation, and regulation of lipid metabolism.
3.4 GSDMD-NT induces NASH model
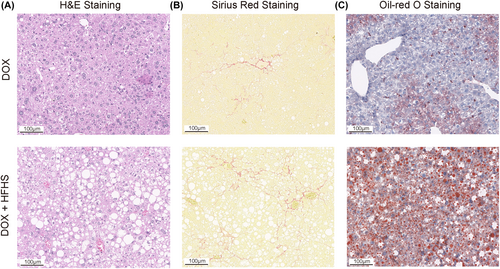
To further develop the modeling potential of GSDMD-NT, we used HFHS to establish a NASH mouse model. After being fed a HFHS diet for 11 weeks, dox-induced GSDMD-NTki/wt × Alb-creki/wt mice exhibited an increase in hepatocyte vacuoles (Figure 4A). Sirius Red staining revealed that liver tissues from dox-induced GSDMD-NTki/wt × Alb-creki/wt mice showed increased collagen deposition (Figure 4B). Additionally, Oil Red O staining demonstrated that liver sections from dox-induced GSDMD-NTki/wt × Alb-creki/wt mice displayed more pronounced steatosis (Figure 4C). Taken together, our data suggested that GSDMD-NT plays a relevant role in NASH pathogenesis.
4 DISCUSSION
Liver fibrosis can result from various chronic liver conditions and causes, including viral hepatitis, alcoholic liver disease, and nonalcoholic steatohepatitis.31-33 Currently, lifestyle interventions are the most effective strategies to manage NAFL and prevent it from progressing to fibrosis. Multiple lines of evidence emphasize the significance of pyroptosis, a form of programmed necrosis, in eliciting robust inflammatory responses and inflicting damage on target organs.34 Upon inflammasome activation, GSDMD is the ultimate executor of pyroptosis, precipitating inflammation, tissue damage, and fibrosis.35-37 We speculated that GSDMD-NT-driven pyroptosis holds promise as a strategy for combatting liver fibrosis.
In the present study, we investigated the functional significance of pyroptosis in hepatocytes through enhancing GSDMD-NT expression. We found that the induction of liver fibrosis in GSDMD-NTki/wt × Alb-creki/wt mice by doxycycline was predominantly driven by GSDMD-NT-mediated pyroptosis. Our data demonstrated that the overexpression of GSDMD-NT create a significant liver injury. These conclusions are consistent with previous studies.23, 38, 39 We observed heightened liver inflammation, increased collagen fiber deposition, and exacerbated steatosis in dox-induced GSDMD-NTki/wt × Alb-creki/wt mice. Moreover, we noted significantly elevated LDH release in the culture supernatant of primary hepatocytes derived from GSDMD-NTki/wt × Alb-creki/wt mice. We confirmed a substantial increase in GSDMD-NT expression in primary hepatocytes. These findings collectively point towards the occurrence of GSDMD-NT-mediated pyroptosis in primary hepatocytes of GSDMD-NTki/wt × Alb-creki/wt mice and underscore the pivotal role played by GSDMD-NT in liver fibrosis pathogenesis.
We further explored the molecular mechanisms underlying the functional role of GSDMD-NT in liver fibrosis. GO enrichment analysis of differentially expressed genes in the GSDMD-NTki/wt × Alb-creki/wt-treated group indicated significant changes in metabolic responses and inflammatory processes. IL-1β is a crucial pro-inflammatory cytokine that plays a pivotal role in driving the pathogenesis of liver inflammation, steatosis, injury, and fibrosis.40-42 MCP-1 stimulates macrophage activation, induces inflammation, and stimulates adipogenesis.43, 44 Therefore, we considered whether GSDMD-NT may influence the secretion of these cytokines within the context of fibrosis. We found that GSDMD-NTki/wt × Alb-creki/wt mice exhibited an increase in hepatic production of IL-1β and MCP-1. Additionally, we assessed the gene expression of liver lipid metabolism. We demonstrated that the overexpression of GSDMD-NT significantly augmented the synthesis of fatty acids and triglycerides (TG).45 The underlying mechanism of liver TG accumulation in fibrosis can be attributed to an enhanced uptake and synthesis of fatty acids and inhibition of fatty acid oxidation. Our data showed that GSDMD-NTki/wt × Alb-creki/wt mice exhibited significantly increased hepatic TG content and steatosis. This increase was associated with increased levels of lipogenic genes, including Fasn, Scd1, Acaca, and Gpam genes. Studies have found that fatty acid synthase can increase excess liver fat and directly regulate inflammation and fibrogenic pathways.46 Hua et al. found that naringenin reduced liver fibrosis and cell senescence and inhibited liver inflammation by regulating the expression of lipid metabolism-related genes (FASN, SCD1, PPARα and CPT1α).47 Another study found that FZHY improved liver fibrosis in rats via altering the metabolic pathways and downregulating the expression of metabolic enzyme genes such as Gs and Acss2.48 More importantly, the researchers found six hub genes involved in the development of chronic schistosomiasis japonica-induced hepatic fibrosis, including ACACA, ACSL1, GPAM, THRSP, PLIN1 and DGAT2.49 Collectively, these findings suggest that GSDMD-NT-induced liver fibrosis may be intricately associated with the regulation of pro-inflammatory cytokine secretion and the expression of genes involved in lipid metabolism.
Farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily, and controls bile acid homeostasis, inflammation in the liver and intestines, liver fibrosis, and cardiovascular disease.50-53 Given its potential impact on liver fibrosis, numerous studies have explored the efficacy of different FXR agonists as potential treatments for fibrosis.54-56 Obeticholic acid (OCA) is widely used in clinical research as an FXR agonist.57 OCA can ameliorate portal hypertension in cirrhotic rats and alleviate liver fibrosis, especially when used in conjunction with apoptosis inhibitors.12, 58 Moreover, OCA has the potential to improve NASH by directly inhibiting the activation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome in macrophages, which subsequently suppresses hepatic lipid accumulation resulting from inflammasome activation.59 We further explored the efficacy of OCA in improving fibrosis. OCA treatment can impede the fibrosis progression, attenuate the activation of HSCs, reduce macrophage infiltration, and downregulate the expression of lipid metabolism related genes.
Targeted therapy may be a novel approach for NAFLD/NASH treatment.60, 61 Mice on a HFHS diet have characteristics similarly to NASH and can be used as a model for the study of human NASH treatments.
5 CONCLUSION
Our data strongly indicate that GSDMD-NT-mediated pyroptosis plays a crucial role in the progression of liver fibrosis. These discoveries provide fresh perspectives on the potential treatment of fibrosis in human patients. In this study, we successfully established a novel mouse model of liver metabolism. By inducing conditional cell death through drugs and dietary interventions, we generated mouse models exhibiting varying degrees of fibrosis and distinct rates of fibrosis progression. These models offer more robust tools for investigating the mechanisms underlying liver fibrosis and the development of therapeutic interventions.
AUTHOR CONTRIBUTIONS
Xue Wang: Conceptualization; data curation; formal analysis; project administration; supervision; writing – original draft; writing – review and editing. Chunyou Ning: Data curation; formal analysis; methodology; resources; software; writing – review and editing. Xingyi Cheng: Formal analysis; investigation; methodology; visualization; writing – original draft. Zhengzhong Wu: Formal analysis; investigation; resources; validation; writing – review and editing. Dongbo Wu: Formal analysis; investigation; methodology; resources; writing – review and editing. Xuemei Ding: Formal analysis; methodology; validation; visualization; writing – review and editing. Cunxiang Ju: Investigation; methodology; supervision; writing – review and editing. Zhihang Zhou: Formal analysis; funding acquisition; resources; software. Lingfeng Wan: Methodology; resources; writing – review and editing. Wei Zhao: Conceptualization; formal analysis; funding acquisition; project administration; supervision; writing – original draft; writing – review and editing. Peiliang Shi: Conceptualization; methodology; resources; writing – original draft.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Cunxiang Ju at the R&D Department of GemPharmatech Co., Ltd. for suggestions and proofreading of this paper. And we thank the Hepatobiliary Surgery Department, West China Hospital, Sichuan University, and the patients who generously donated samples for biomedical research. We thank Gabrielle White Wolf, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript. This research was funded by Sichuan Province Science and Technology Program (2024NSFSC0577, 2021YFG0316).
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (82174292); Key Project of Jiangsu Provincial Administration of Traditional Chinese Medicine (ZD202312) and Natural Science Foundation of Laboratory Medicine School in Chengdu Medical College (JYZK202203). This research was funded by Sichuan Province Science and Technology Program (2024NSFSC0577 and 2021YFG0316).
CONFLICT OF INTEREST STATEMENT
The authors have declared that no conflict of interest exists.
6 ETHICS STATEMENT
Medical ethics committee approval of specimen collection was obtained from Nanjing University of Chinese Medicine's Affiliated Hospital (approval number: 2022NL-014-02), and written informed consent was obtained from patients. All experimental procedures involving animals in this study were conducted in accordance with protocols that were thoroughly reviewed and given approval by the Institutional Animal Care and Use Committee (IACUC) at GemPharmatech Co. Ltd, with a reference number of (CDAP20211018-1#).




