Single-cell sequencing reveals the features of adaptive immune responses in the liver of a mouse model of dengue fever
Abstract
Background
Dengue fever, an acute insect-borne infectious disease caused by the dengue virus (DENV), poses a great challenge to global public health. Hepatic involvement is the most common complication of severe dengue and is closely related to the occurrence and development of disease. However, the features of adaptive immune responses associated with liver injury in severe dengue are not clear.
Methods
We used single-cell sequencing to examine the liver tissues of mild or severe dengue mice model to analyze the changes in immune response of T cells in the liver after dengue virus infection, and the immune interaction between macrophages and T cells. Flow cytometry was used to detect T cells and macrophages in mouse liver and blood to verify the single-cell sequencing results.
Results
Our result showed CTLs were significantly activated in the severe liver injury group but the immune function-related signal pathway was down-regulated. The reason may be that the excessive immune response in the severe group at the late stage of DENV infection induces the polarization of macrophages into M2 type, and the macrophages then inhibit T cell immunity through the TGF-β signaling pathway. In addition, the increased proportion of Treg cells suggested that Th17/Treg homeostasis was disrupted in the livers of severe liver injury mice.
Conclusions
In this study, single-cell sequencing and flow cytometry revealed the characteristic changes of T cell immune response and the role of macrophages in the liver of severe dengue fever mice. Our study provides a better understanding of the pathogenesis of liver injury in dengue fever patients.
1 INTRODUCTION
Dengue fever is an acute infectious disease caused by dengue virus (DENV), which is transmitted by mosquitoes and infects approximately 400 million people annually in more than 130 countries.1 In 2023, more than 5 million cases of dengue fever were reported in 80 countries around the world, resulting in more than 5,000 deaths due to severe dengue, mainly distributed in South Asia, Southeast Asia, and other regions. Thus, dengue fever poses a considerable threat to global public health.2 However, there is currently no targeted treatment for dengue fever. Current clinical treatment of dengue fever only focuses on symptomatic support, such as fluid rehydration and platelet transfusion.3, 4
Liver involvement,5 including elevated aminotransferase (45%–97%), hepatomegaly (28%–72%)6 and acute liver failure, is the most common complication in dengue fever patients, with a fatality rate of 58.3%–68.3%.7 Clinical studies have shown that elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels can indicate severe disease manifestations and poor clinical outcomes. Most deaths from dengue fever are associated with acute liver failure. A large number of reports have shown hyperemia, swelling, steatosis and necrosis in the liver of patients who died from dengue fever.8, 9 However, the pathological mechanism of liver injury caused by DENV infection is still unclear, hindering the development of effective treatments for dengue fever.
Adaptive immune involvement in liver damage has been observed in a variety of infectious diseases.10 The number of HCV-specific CD8+ T cells in the liver and circulating in patients with chronic hepatitis C is not significantly reduced, but their proliferative capacity, IFN-γ secretion and cytotoxicity are significantly impaired, limiting their ability to clear HCV.11, 12 Extensive data also indicate increased Treg cell numbers in patients with chronic hepatitis C, which can directly inhibit HCV-specific CD8+ T cells and exert immunosuppressive effects by altering the innate immune system.13 The number of Th17 cells in the blood and liver is significantly increased in patients with hepatitis B, along with elevated levels of IL-17 and IL-22 in the blood. Moreover, Th17 cells are associated with the severity of liver disease in patients with chronic hepatitis B, suggesting that Th17 cells may be involved in the disease process of acute and chronic HBV infection.14 Yellow fever virus (YFV) is a flavivirus that causes severe liver injury.15 Studies have shown that CTL migration from lymph nodes to tissues, especially to the liver, is mediated by CCR5, and overactivation of effector T cells is associated with liver injury in YFV infection.16 However, the role of host adaptive immunity in liver injury caused by severe dengue remains unclear.
In this study, based on dengue fever mouse models with severe or mild liver injury established earlier in our laboratory, adaptive immune cells in the liver were analyzed in detail through single-cell sequencing (scRNA-seq) to understand the characteristic changes in adaptive immunity in the liver during dengue disease, providing a theoretical basis for the clinical occurrence and development of severe dengue liver injury.
2 METHODS
2.1 Virus strains
The New Guinea C (NGC) strain of DENV was obtained from the American Type Culture Collection (ATCCVR-1584), and the N10 strain used in our study was adapted from the NGC strain through several passages in mice by our group.
2.2 Mouse and modeling
Six- to eight-week-old clean-grade C57BL/6 type I IFN receptor knockout mice (IFNAR−/− mice) were purchased from Beijing Huafukang Biotechnology Co., Ltd (SCXK (JING)2019-0008, Beijing, China; 9 mice in total weighing 18–22 g). The mice were kept in the SPF-grade animal house of the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (ILAS, CAMS), provided with sufficient feed, water and with normal day and night rhythms. For viral infection, 9 type IFNAR−/− mice were randomly and equally divided into 3 groups—the uninfected group, the NGC-infected group, and the N10-infected group—and the NGC, N10 groups were infected with the NGC strain (1.2 × 106 TCID50/mL) or the N10 strain (3 × 106 TCID50/mL) by tail vein injection at 0 dpi, respectively. Daily observation data were recorded during the infection period, and at 6 dpi, the animals were sacrificed, and the appropriate amount of liver tissue was collected for single-cell sequencing. All animal experiments were conducted according to the animal welfare regulations of the ILAS, CAMS (IACUC: WW22001).
2.3 Flow cytometry
For blocking antibodies, 50 μL anticoagulated whole blood +50 μL PBS was added to each tube in the flow tube rack and 1 μL dead/live Zombie Aqua was added for every 1–10 × 106 cells/mL. The tubes were gently vortexed a few times to resuspend the cells, and incubated at 4°C for 30 min. Then 1 mL cell staining buffer was added to each tube, and the tubes were vortexed gently a few times to resuspend the cells, and then centrifuged at 300× g for 5 min at 4°C. Then 100 μL of the configured CD16/32 blocking antibody was added to each tube, and the tubes were vortexed gently for a few times to resuspend the cells, and incubated at room temperature for 20 min in the dark.
For extracellular staining, 100 μL of the prepared corresponding epistaining antibody was added to each tube, and the tubes were vortexed gently for a few times to resuspend the cells, and incubated at room temperature for 30 min in the dark. Then 1 mL cell staining buffer was to each tube, and the tubes were again vortexed gently a few times to resuspend the cells, and centrifuged at 300× g for 5 min at 4°C before discarding the supernatant.
For red blood lysis, 1 mL BD red blood cell lysate was added to each tube, followed by 1 mL PBS after 2–3 min to stop red blood lysis, and the tubes were centrifuged at 300× g for 5 min at 4°C, before discarding the supernatant.
For intranuclear staining, 1 mL 1× True-Nuclear suspension Fix buffer resuspension cells was vortexed and incubated at room temperature without light for 45–60 min. Without washing, 2 mL True-Nuclear powder Perm Buffer (1×) was directly added and the tubes were centrifuged at 300–400× g centrifugal for 5 min, before discarding the supernatant. Then 2 mL True-Nuclear resin Perm Buffer (1×) was again added, and the tubes were centrifuged at 300× g for 5 min, before discarding the supernatant. The cells were re-suspended in True-Nuclear goat Perm Buffer (1×), and the corresponding antibodies were added according to the experimental design and antibody instructions, to give a total reaction volume of 100 μL. This was incubated for 30 min at room temperature without light and then 2 mL True-Nuclear resin Perm Buffer (1×) was added, the tubes were centrifuged at 300× g for 5 min, and the supernatant was discarded. Cell Staining Buffer (2 mL) was then added, the tubes were centrifuged at 300× g for 5 min, and the supernatant was discarded.
To prepare for flow detection, 300 μL Cell Staining Buffer was added. The liver tissue is digested and decomposed by collagenase IV and filtered through a 70 nm filter to prepare a single cell suspension. The staining procedure was the same as that used for blood.
2.4 10× Genomics Chromium single-cell RNA sequencing data processing (all operations and analyses were performed by 10× Genomics)
For cell separation and nuclei extraction, the samples were placed in dry ice and transferred to the indoor test bench. Tweezers were used to remove the tissue from the cryogenic storage tube. The tissue was then transferred it to a grinder containing dissolution buffer, and ground thoroughly until there was no obvious tissue mass by visual inspection. The ground suspension was collected and filtered through a 40 μm filter and then centrifuged (500× g, 5 min, 4°C). The supernatant was removed and the pellet was resuspended in storage buffer. The cell nuclei were stained with NucBlue Live ReadyProbes reagent (Thermo Fisher Scientific, Waltham, MA, USA), and Hoechst-positive mononuclear nuclei were purified by inflow fluorescence-activated cell sorting. Verification of cell nuclei purification and integrity was carried out under a microscope. Basic statistics on the quality of the raw reads were obtained using Fastp. Using default parameters, the raw reads were demultiplexed and mapped to the reference genome using the 10x Genomics Cell Ranger pipeline (cellranger-6.0.0). Cellranger aggr aggregates the output of multiple runs of cellranger count, normalizes these runs to the same sort depth, and then recalculates the feature barcode matrix and its combined data analysis. The Seurat package was used for normalization, dimensionality reduction, clustering, and differential representation of the data. Canonical correlation analysis (CCA) was performed by the Seurat calibration method.
For cell subgroup annotation, to annotate each cluster to a specific immune cell type, we selected several classical markers of immune cells and used scatterplots and violin plots to annotate the cell types. The following genes were used for cell type annotation: TCF7, LEF1 (naïve T cells); GZMA, GZMB (cytotoxic CD8+ T cells); CTLA4, TIGIT (Treg); KLRC1, NCR1 (NKT); IL2RA, TBX21 (Th1); IL-17, CXCR6 (Th17); MS4A1 (mature undifferentiated B cells); and CD38 (plasma cells).
For gene function annotation, DEG, Gene Ontology (GO), and KEGG enrichment analysis of three sets of samples were performed using the clusterProfiler R package Reactome pathway-based marker gene analysis in the ReactomePA R package. The screening criteria for differentially expressed genes were p_val_adj <0.05, |avg_log2FC| >0.5.
Intercellular communication analysis based on known ligand–receptor pairs in cell types was performed using CellChat (version 1.1.0). Standardized gene expression matrices and cell type labels generated by Seurat were used as input to CellChat. For the main analysis, the core functions ‘computeCommunProb’, ‘computeCommunProbPathway’ and ‘mergeCellChat’ were applied using standard parameters. For ‘mergeCellChat’, the ‘computeNetSimilarityPairwise’ function was used to compute the similarity between pathways and cluster pathways into different groups based on functional similarity.
2.5 Quantitative and statistical analysis
Statistical analysis was performed using R (version 3.6.1). The Wilcoxon rank sum test and Student's t test were used in this study. The data from flow cytometry analysis had normal distribution, and an independent sample t test was used. GraphPad Prism 8.0 version (GraphPad Software, Inc., La Jolla, CA, USA) was employed to analyze and draw the relevant figures. p < 0.05 was considered to indicate a statistically significant difference.
3 RESULTS
3.1 T-cell numbers are slightly reduced in severe liver injury caused by DENV infection
In preliminary research, 6- to 8-week-old IFNAR−/− mice were infected with dengue-2 virus (NGC or N10 strain) to establish a liver injury model. The NGC-infected mice showed mild liver injury, and the N10-infected mice showed severe liver injury (Figure S1).
To determine the characteristics of adaptive immunity in the liver of dengue fever, single-cell suspensions were obtained by isolating liver tissues from the uninfected, NGC and N10 groups of mice. The final dataset contained a single-nucleus transcriptome merged from 9 samples, and bioinformatics data analysis was performed (Figure 1A). Using uniform manifold approximation and projection (UMAP), 10 T-cell clusters (0–9) and 5 B-cell clusters (0–4) were identified (Figure 1B). To understand the characteristics of adaptive immunity related to the occurrence and development of disease, the distributions of T and B cells among the uninfected, mildly infected and severely infected groups were compared. There were greater proportions of T and B cells in the mild and severe liver injury model groups than in the uninfected group (Figure 1C). Compared to the uninfected group (60.27%), the infected group contained a lower percentage of T cells. Interestingly, the percentage of T cells in the severe group (87.15%) was lower than that in the mild group (92.09%) (Figure 1D).
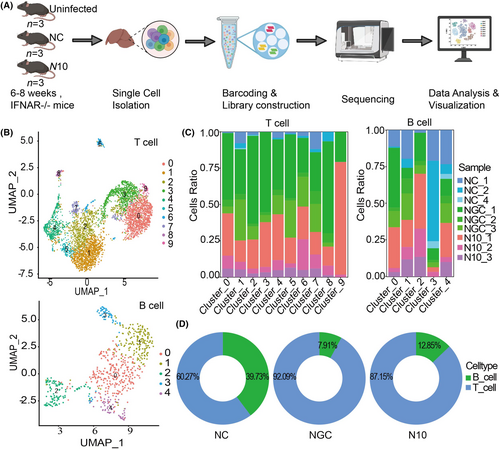
Together, the above results show that adaptive immunity, especially cellular immunity, was significantly activated in the liver after DENV infection. The possible reasons are increased recruitment or clonal expansion of immune cells, especially T cells, in the liver in response to DENV infection and liver injury. However, the proportion of T cells in the severe group was slightly lower than that in the mild group. We aimed to further investigate the relationship between T-cell function and the degree of liver injury.
3.2 Naïve T cells was significantly activated and differentiated into effector T cells in the severe group
To characterize the T-cell immune response accompanying liver injury in dengue fever, we identified 6 subtypes of T cells and a cluster of undefined T cells (Figure 2A), including cytotoxic T Lymphocyte (CTL), naïve T cells (Tn), helper T cells (Th) 1, helper T cells 17, natural killer T cells (NKT), and regulatory T cells (Treg) (Figure 2A,B).
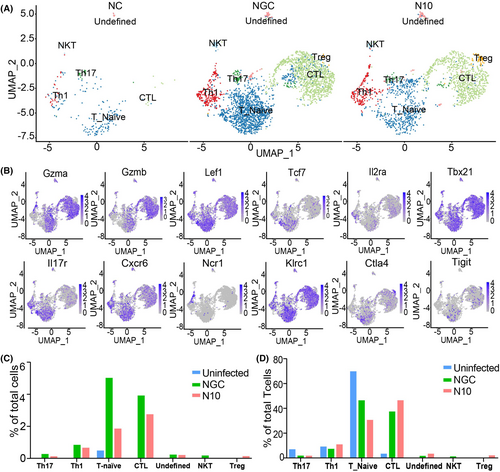
To determine the abundance of T-cell subtypes in the liver, the proportions of T-cell subtypes at the total cell level and at the T-cell level were compared. Comparing the percentage of total T-cell subsets in the severe group with the mild group showed that only the number of Treg cells increased, while the number of CTL, Th17, NKT, and other cell subsets decreased (Figure 2C). The above results once again proved that the number of T cells was reduced in the severe liver injury group. By comparing the percentages of each T-cell subpopulation within the overall T-cell population between the three groups, we found that as the severity of liver damage increased there was a decrease in the percentages of Tn (from 71.24% to 47.13%) and Th17 (from 8.41% to 2.44%) T-cell subtypes, and an increase in the percentages of CTLs (from 4.87% to 47.13%) and Treg (from 0.00% to 2.67%) T-cell subtypes (Figure 2D), suggesting that as the degree of liver injury increased, more naïve T cells were activated and differentiated into effector T cells, such as CTLs, in the liver to exert an immune function.
3.3 Decreased expression of immune-related signaling pathway genes in CTLs in the severe group
Through the above analysis, we further focused on the gene function changes in each T-cell subpopulation, especially CTLs and Tregs, between the mild and severe groups. For CTLs, 42 upregulated genes and 48 downregulated genes were differentially expressed (p_val_adj <0.05, ∣avg_log2FC∣ >0.5) in the severe group compared with the mild group. The expression of BCL2 Apoptosis Regulator (Bcl-2), Inter-Alpha-Trypsin Inhibitor Heavy Chain 3 (Itih3), and Hepcidin Antimicrobial Peptide (Hamp) increased, and the expression of the erythroid differentiation regulator (Erdr1), epidermal growth factor receptor (Egfr), and fibroblast growth factor (Fgf13) genes decreased (Figure 3A).
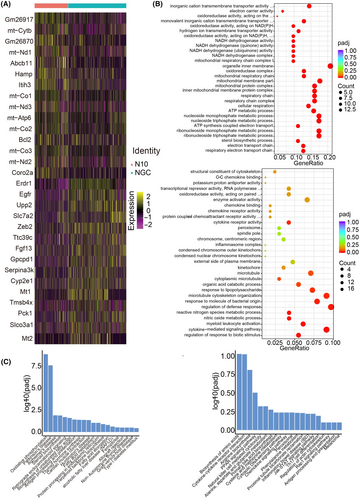
The above results showed that CTL immune-related signaling pathways were decreased in the liver in the severe group compared with those in the mild group, suggesting that CTL immune function was negatively correlated with the degree of liver injury.
Furthermore, Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were performed on different T-cell subtypes to explore functional changes in T cells. The results of GO analysis showed that immune-related signaling pathways such as cytokine-mediated signaling pathways, regulation of defense translation, and cytokine receptor activity were decreased in the severe group compared with the mild group. In contrast, cellular metabolism-related signaling pathways such as nucleotide metabolism and the mitochondrial inner membrane pathway were increased in the severe group compared with those in the mild group (Figure 3B). KEGG analysis revealed that cytokine-receptor interactions and amino acid biosynthesis were decreased in CTLs, while oxidative phosphorylation and Parkinson's disease pathways were increased (Figure 3C). The GO and KEGG results for Tn also showed that immune-related signaling pathways associated with T cells were decreased in the severe group (Figure S2A,B). The number of Tregs and Th17 cells was too small to perform pathway enrichment analysis.
We investigated the differences in the number and function of effector T cells between the mild and severe groups. With the exception of Tregs, the number of other T-cell subpopulations was lower in the severe group than in the mild group. Moreover, the CTL and Tn immune functions of the severe group were weakened. Tregs are closely related to Th17 cells; they regulate each other in the process of differentiation and are essential for maintaining liver immune homeostasis, and a Th17/Treg imbalance leads to liver injury.13 Therefore, we hypothesized that the decrease in the number of T cells in the critical group might be related to the Th17/Treg imbalance.
3.4 Interaction between multiple T-cell and macrophage subtypes in the severe group
To further explore the reasons for the changes in the number and function of effector T cells, we explored local cell–cell interactions in severe or mild liver injury groups. First, we assessed differences in the number of specific interactions via CellChat. Numerous specific interactions were predicted between all T cell, monocyte and macrophage subtypes. In both the mild and severe groups, Th17 cells were characterized by a high number of specific interactions with other cell subtypes. However, the interactions between Th17 cells and Kupffer cells in the mild group were significantly stronger than those in the severe group, and the interactions between Th17 cells and Tregs in the severe group were significantly stronger than those in the mild group (Figure 4A,B).
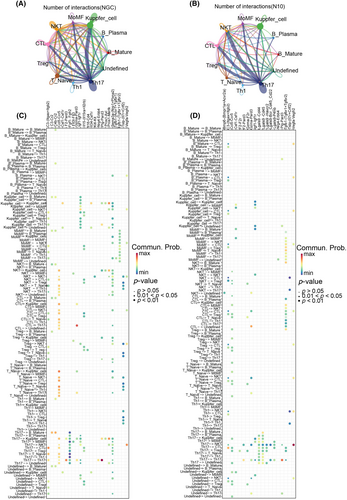
Furthermore, to understand the main signaling molecules involved in cell communication between the two groups, we analyzed the receptor pairs involved in cell-to-cell interactions via CellChat. In the mild liver injury group, among the T cells and macrophages, some immunostimulatory interactions (CCL5/CCR5 and C3-(Itgax+Itgb2)) were enhanced. A very different scenario was observed in critical liver injury. Among the numerous interactions between macrophages and CTLs, we detected immune-inhibitory interactions (TGF-β1-(TGF-βr1 + TGF-βr2)) (Figure 4C,D), which may induce CTL dysfunction. In brief, macrophages and T-cell subsets interact frequently, and macrophages may affect the number and immune function of CTLs in the severe group.
3.5 Macrophages may participate in liver injury by upregulating the TGF-β pathway
To explore the signaling pathways through which different subsets interact, we first inferred intercellular communications for mild or severe datasets separately based on CellChat. The common cell populations between the mild and severe datasets were used. As a result, the signaling pathways associated with inferred networks from the mild and severe groups were mapped onto a 2-dimensional manifold and clustered into different groups. We identified 4 pathway groups for the mild group and 6 pathway groups for the severe group (Figure 5A). According to the difference in signaling pathway expression between the two groups, we focused on the role of different cell subsets in the TGF-β, CCL, and complement signaling pathways (Figure 5B).
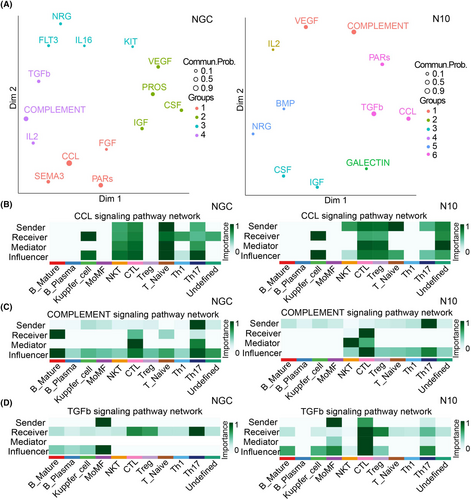
Tn is the leading sender of Kupffer cells in the CCL pathway in the mild and severe groups (Figure 5B). Previous ligand analysis revealed that the CCL5/CCR5 interaction was enhanced. Clinical studies have shown that compared with that in patients with mild disease, the expression of CCR5 in the serum of patients with severe disease is increased, and the expression of CCR5 is positively correlated with viral load.17 Hence, we speculated that the upregulation of the CCL signaling pathway may mediate liver injury.
In the mild group, Th17 cells act mainly as transmitters of mature B cells via the complement signaling pathway. In the severe group, Th17 cells mainly acted on CTLs (Figure 5C). There are many kinds of complement deposition in liver samples from patients with fatal dengue fever,18 suggesting that the role of the complement system in severe dengue fever liver injury is also worthy of attention.
In the TGF-β pathway, macrophages in the mild group mainly acted on Th17 cells, while macrophages in the severe group significantly enhanced their effects on CTLs and Tregs (Figure 5D). TGF-β is necessary for the differentiation of Th17 and Treg subsets.19 Th17 cells and Tregs regulate each other during differentiation, which is very important for maintaining liver immune homeostasis. In the severe group, the effect of macrophages on Tregs through the TGF-β pathway may lead to an imbalance in Th17/Treg cells and induce liver injury.
Compared with those in the mild group, the TGF-β signaling pathway, in which macrophages act on CTLs, in the severe group was significantly increased. TGF-β can exert immunosuppressive effects by inhibiting T-cell activation and proliferation. M2 macrophages, as anti-inflammatory macrophages, further release anti-inflammatory factors such as TGF-β and IL-10.20 The above results suggest that the pathological mechanism of liver injury in severe dengue fever is very complex and that multiple signaling pathways are involved. Macrophages may participate in liver injury by upregulating the TGF-β pathway to induce Th17/Treg imbalance and weaken the immune function of CTLs; upregulation of the CCL pathway and complement pathway may also be involved in liver injury, but the specific mechanism involved remains to be explored.
3.6 More mature B cells were activated and transformed into plasma cells in the severe group
Thus, we aimed to more comprehensively understand the adaptive immune response in the liver in dengue fever patients. The changes in the number and function of B cells were analyzed briefly. First, we defined Clusters 0, 1, 3, and 4 as mature B cells (CD19 and CD38) and Cluster 2 as plasma cells (CD27 and Enpp1) according to characteristic genes (Figure 6A, C). To understand the changes in the abundances of the two kinds of B-cell subsets after DENV infection and liver injury, we analyzed the proportions of B-cell subsets in the three groups of total B cells. The results indicated that the proportion of mature B cells decreased and that the proportion of plasma cells increased in the severe group. These findings suggested that more mature undifferentiated B cells transform into plasma cells after virus infection and liver damage (Figure 6B).
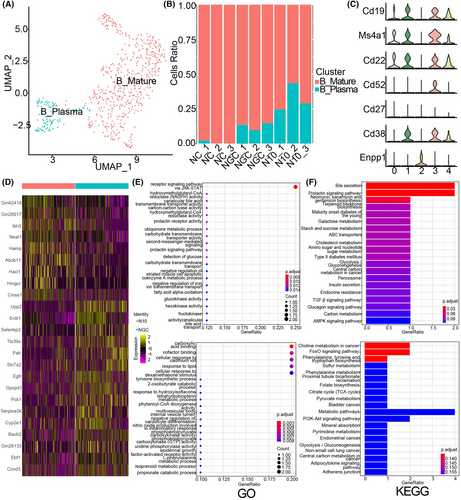
To determine the changes in DEGs between the mild group and severe group, through the analysis of DEGs (p_val_adj <0.05, |avg_log2FC| >0.5), we obtained 9 upregulated genes and 16 downregulated DEGs, among which Itih3, Hamp, Abcb11, Hacl1, Hmgcr and others were upregulated. Erdr1, Upp2, Gpcpd1, Ttc39c, and Egfr were downregulated (Figure 6D).
Additionally, we investigated the functional changes in B cells after DENV infection. Functional enrichment analysis of different B-cell subsets was performed in the mild and severe groups. The results of GO analysis showed that the JAK–STAT signaling pathway was increased in mature and undifferentiated B cells from severe group mice. Moreover, carboxylic acid binding and cofactor binding were decreased (Figure 6E). The results of KEGG analysis showed that choline metabolism, FoxO, and other signaling pathways were decreased, while bile secretion and prolactin were increased (Figure 6F).
The above results show that more mature undifferentiated B cells in the liver are transformed into plasma cells after DENV infection, and the upregulation of the JAK–STAT signaling pathway in mature undifferentiated B cells may be related to the activation of the immune response.
3.7 The proportion of CTLs in liver and blood increased significantly in the severe group
Based on the above single-cell data analysis results, in order to more intuitively understand the changes of immune responses of T cells and macrophages in dengue mouse models with different degrees of liver injury, we conducted flow cytometry analysis of the changes of macrophages and T cell subtypes, especially CTLs, in liver and blood of the normal control group, mild liver injury group and severe liver injury group. Flow results showed that on the 6th day after infection, the proportion of GZMB+T cells among CD8+T cells in the liver of the severe group was about 43.7%, and only 22% in the mild group (Figure 7A). GZMB+T cells accounted for 47.25% of CD8+ T cells in the blood of the severe group, which was also significantly higher than in the mild group (23.6%) (Figure 7B). The above results indicated that the number of CTLs in the severe group was significantly higher than that in the mild group, which was consistent with the previous single-cell sequencing results. However, the changes in the number of macrophages were far less significant than the changes in CTLs. It is worth noting that both in blood and liver, the pro-inflammatory M1 macrophages in the severe group were slightly lower than those in the mild group (Figure 7C), and the anti-inflammatory M2 macrophages in the severe group were slightly higher than those in the mild group (Figure 7D), indicating that macrophages tended to be M2-type polarized.
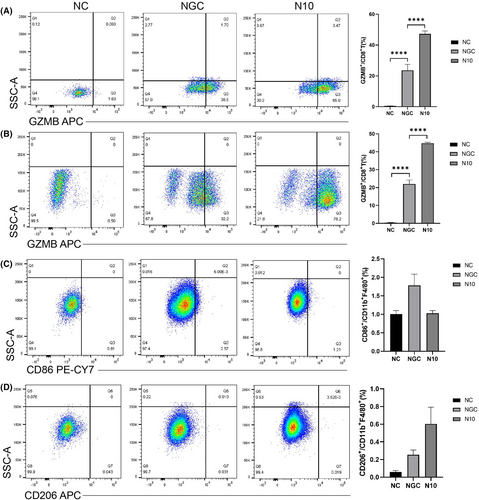
In summary, the results of flow analysis confirm that CTLs are significantly activated in the severe group, and CTLs exert a cell-killing function by releasing granzyme B, which may be one of the reasons for the severe liver injury in the severe group. At the same time, macrophages in the severe group tended to be M2-type polarized, which may be related to the excessive inflammatory response in the mice in the middle-to-late stage of infection in the severe group.
4 DISCUSSION
The liver is the most commonly affected organ in severe dengue,5 but little is known about the pathogenesis of dengue liver injury, especially the role of the adaptive immune response. In this study, we performed the single-cell sequencing of the livers of dengue-infected mice with different degrees of damage, revealing the adaptive immune response characteristics of the livers of mice with severe dengue liver injury and providing a theoretical basis for a better understanding of the pathogenesis of dengue liver injury.
T cells were dysregulated in the critical group. We observed that, compared with the mild group, the severe group not only showed a decrease in the number of T cells with good effector functions but also exhibited a decrease in immune-related signaling pathways. Interestingly, Tregs were the only cell subtype that increased in the severe group. This result is consistent with the latest clinical research reports, and multiple studies on severe dengue have shown that Tregs are also increased in the PBMCs of patients.1, 21 Notably, there is a close and complex relationship between Tregs and Th17 cells, and Tregs can directly inhibit the function of antigen-presenting cells and release anti-inflammatory factors such as IL-10 and TGF-β to inhibit the activation and proliferation of T cells.22 Th17 cells, as T helper cells, secrete the proinflammatory factors such as IL-17 and IL-21 to activate CTLs to exert their proinflammatory effects.23 Overall, these findings showed that the differentiation of T cells in the liver of the severe group was disrupted, and the immune response of the host against virus infection could not be carried out normally.
The mechanism of T-cell imbalance is complex. Given the important role of macrophages in liver injury caused by severe dengue, we found that macrophages and T-cell subsets interact frequently and that macrophages may inhibit the immune function of CTLs in the severe group. Moreover, we revealed that the TGF-β, CCL, and complement signaling pathways play important roles in mediating cell–cell communication.
Our data showed that macrophages in the severe group mainly acted on CTLs and Tregs through the TGF-β signaling pathway. TGF-β mainly exerts immunosuppressive effects. The TGF-β signaling pathway plays a key role in promoting the polarization of M2 macrophages.24 As a necessary cytokine for Th17 and Treg differentiation, TGF-β can promote Th17 and Treg differentiation in a concentration-dependent manner. TGF-β can also inhibit the activation of naïve T cells by antigen-presenting cells and inhibit the proliferation and differentiation of naïve T cells into CTLs. Therefore, we speculate that macrophages in the livers of severe patients not only regulate their own M2 polarization through TGF-β but also act on CTLs and Tregs to cause T-cell dysfunction. On the one hand, it exerts its immunosuppressive function by promoting Treg differentiation, and on the other hand, it inhibits its immunological function by directly acting on CTLs.
The role of CCR5 in severe dengue has also long been reported. Clinical studies have shown that CCR5 expression is increased in severe patients compared with mild patients, and CCR5 expression is positively correlated with viral load,17 suggesting that CCR5 may mediate liver damage by promoting viral infection. Additionally, several complement components, including C9 deposits in hepatocytes, Kupffer cells, and macrophages, were found to be deposited in the liver tissues of children with fatal dengue fever, and C1q, C3b, and C9 deposits were found in germinal centers.18 These findings suggest that complement system activation is also involved in mediating severe dengue liver injury.
Nevertheless, there are also some limitations to our study. We observed evidence of T-cell involvement in liver damage caused by severe dengue, but the specific underlying mechanisms still need to be described in depth. In addition, although severe clinical samples are extremely difficult to obtain, further validation studies involving clinical liver injury samples are still needed.
5 CONCLUSIONS
In conclusion, we used single-cell transcriptomics and flow cytometry detection to characterize the adaptive liver immune response to dengue liver injury. We observed marked changes in immune cell composition, phenotypes and immune cross-talk during DENV infection and identified several distinguishing immunological signaling pathways involved in mild vs. critical liver injury. We observed that CTL was significantly activated in the severe liver injury group, but the immune function-related signal pathway was down-regulatd. The reason may be that the excessive immune response in the severe group in the late stage of DENV infection induced macrophage polarization to the M2 type, and macrophages then inhibited T cell immunity through the TGF-β signaling pathway. Also, the increased proportion of Treg cells suggested that Th17/Treg homeostasis was disrupted in the livers of severe liver injury mice. The above findings provide a theoretical basis for revealing the pathogenesis of severe dengue liver injury.
AUTHOR CONTRIBUTIONS
Yizhen Yuan was involved in experimental manipulation, data analysis and manuscript writing. Qian Chen was involved in project design and experimental manipulation. Zhe Li, Dan Li, Fangzhou Cai were involved in experimental manipulation or data analysis. Wei Wang was involved in project design, data analysis, fund support, team supervision and manuscript reviewing and approving.
ACKNOWLEDGMENTS
We extend our gratitude for the support provided by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-1-035 and 2022-I2M-1-011).
FUNDING INFORMATION
This work was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (grant no: 2021-I2M-1-035, 2022-I2M-1-011).
CONFLICT OF INTEREST STATEMENT
There is no conflict of interests.
ETHICS STATEMENT
All experiments associated with authentic virus were conducted within a biosafety level 2 (BSL-2) facility. The animal procedures conducted in this study were approved by the Institute of Laboratory Animal Science, CAMS & PUMC (approval no: WW22001).




