Causal relationship between lipid profile and muscle atrophy: A bi-directional Mendelian randomization study
Abstract
Background
The aim of this study was to analyze the bi-directional causal relationship between lipid profile and characteristics related to muscle atrophy by using a bi-directional Mendelian randomization (MR) analysis.
Methods
The appendicular lean mass (ALM), whole body fat-free mass (WBFFM) and trunk fat-free mass (TFFM) were used as genome-wide association study (GWAS) data for evaluating muscle mass; the usual walking pace (UWP) and low grip strength (LGS) were used as GWAS data for evaluating muscle strength; and the triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), apolipoprotein A-1 (Apo A-1), and apolipoprotein B (Apo B) were used as GWAS data for evaluating lipid profile. For specific investigations, we mainly employed inverse variance weighting for causal estimation and MR-Egger for pleiotropy analysis.
Results
MR results showed that the lipid profile predicted by genetic variants was negatively correlated with muscle mass, positively correlated with UWP, and was not causally correlated with LGS. On the other hand, the muscle mass predicted by genetic variants was negatively correlated with lipid profile, the UWP predicted by genetic variants was mainly positively correlated with lipid profile, while the LGS predicted by genetic variants had no relevant causal relationship with lipid profile.
Conclusions
Findings of this MR analysis suggest that hyperlipidemia may affect muscle mass and lead to muscle atrophy, but has no significant effect on muscle strength. On the other hand, increased muscle mass may reduce the incidence of dyslipidemia.
1 INTRODUCTION
Muscle, as the most abundant tissue in the human body, not only controls movement but also participates in a variety of life activities like breathing and feeding.1 Loss of muscle volume and mass caused by diseases such as diabetes mellitus (DM), chronic kidney disease (CKD), and Alzheimer's disease (AD) is referred to as muscle atrophy and is characterized by a decrease in the cross-sectional area of muscle fibers and muscle protein content.2 Muscle atrophy can lead to decreased muscle function and quality of life, and can even lead to death.2, 3
Lipid profile is considered to be a risk factor for diseases including coronary heart disease and hypertension.4 In addition, disorders of lipid metabolism may affect various muscle tissues including skeletal and cardiac muscles.5 Several observational studies have provided evidence for a significant association between muscle mass/strength and lipid profile.6, 7 For example, a cross-sectional survey of 3468 adults in Switzerland showed that increased grip strength (GS) was significantly associated with increased low density lipoprotein cholesterol (LDL) in men and with decreased triglyceride (TG) in women.8 Moreover, elevation of total cholesterol (TC) was also found to be significantly associated with increased GS in men.9 However, the results of another observational study of 1015 Korean adults reported that an increase in GS was significantly associated with a decrease in LDL and TC.10 The conflicting results of these studies may be attributed to the possibility of confounding factors and reverse causation.
A number of recent randomized controlled trials (RCTs) have shown significant increases in high density lipoprotein cholesterol (HDL) concentrations following muscle exercise and protein supplementation.11, 12 Some other RCTs have further demonstrated that muscle exercise and dietary supplementation can significantly reduce TG, TC, and LDL levels.13, 14 Although these studies explored the effects of muscle exercise and dietary supplementation on lipid profile, they lacked direct measurement of indicators such as muscle mass and muscle strength and the results were inconclusive. Moreover, the limited duration of intervention, a weakness of the study in general, did not allow information on potential long-term side effects, which might have led to biased results. Therefore, the causal relationship between lipid profile and muscle atrophy remains uncertain.
Mendelian randomization (MR) is an epidemiological research design that uses genetic variation to determine causal effects between exposures and outcomes.15 Gamete formation is a process of obtaining genetic variants from the parental generation by following the Mendelian laws of randomization, so the obtained genetic variants are generally independent of confounding factors.15 It has been revealed that specific genetic variants can often affect specific exposure factors.15 Therefore, by analyzing the effect of carrying a specific genetic variant on an outcome, the causal relationship between a specific exposure and the outcome can be determined.15 MR analysis is not subjected to the problems of reverse causal associations and confounding factors that exist in traditional observational studies.16 For RCTs, MR analysis not only circumvents conditions, difficulties, and ethical constraints, but will also help generate design ideas.16
In this study, we conducted a bi-directional two-sample MR using specific genetic variants from genome-wide association study (GWAS) datasets as instrumental variables (IVs) to evaluate the potential causal relationship between lipid profile and characteristics associated with muscle atrophy. Our findings were expected to provide new insights into the prevention and treatment of dyslipidemia and muscle atrophy.
2 METHODS
2.1 Study design
In MR analysis, the specific genetic variables used as IVs must fulfill three major assumptions: (1) IVs are strongly associated with exposures; (2) IVs are not associated with confounders; (3) IVs can only affect outcomes through exposures, not directly.15 In the present MR study (see Figure 1. for the specific MR study design), the bi-directional MR analysis of lipid profile and muscle atrophy was performed using GWAS data. In forward MR analyses, lipid profile is the exposure, while muscle atrophy is the outcome. In reverse MR analyses, muscle atrophy is the exposure, while lipid profile is the outcome.
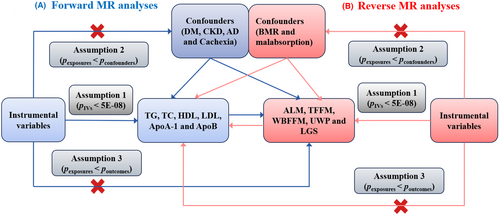
2.2 Characteristics associated with muscle atrophy
Muscle atrophy is usually not directly measurable, which means its extent can only be assessed by selected data related to muscle mass. Appendicular lean mass (ALM) has been recognized as an effective indicator for the loss of muscle mass and strength.17 It can be measured using dual-energy X-ray absorptiometry or bioelectrical impedance analysis and should be adjusted for gender, age, height and fat mass.18 Whole body fat-free mass (WBFFM) and trunk fat-free mass (TFFM) are also deemed effective in assessing the degree of muscle atrophy, but due to involvement of soft tissue mass other than fat, they are not as accurate as the ALM.19, 20
Muscle atrophy is defined as reduction in muscle mass and volume. Indicators of muscle weakness such as GS and usual walking pace (UWP) can also be used to reflect muscle health,21, 22 and low grip strength (LGS) can predict future morbidity and mortality.22 Therefore, LGS and UWP were also selected as investigation factors in this study. Specifically, GS was assessed by a hydraulic hand dynamometer using a Jamar 100 105, and was expressed in absolute (kilograms) and relative (kilograms divided by body weight) units.21 UWP was obtained by questionnaire.21
2.3 Data sources
The open GWAS database established by the MRC Integrated Epidemiology Unit provides the majority of summary-level data used in this study.23 The R package was used to access the application programming interface to download data for MR analysis. More specifically, the ALM data was derived from a GWAS dataset conducted on 450 243 UK Biobank participants.17 The TFFM, WBFFM, and UWP data were derived from the GWAS datasets conducted on 454 508, 454 850 and 335 349 UK Biobank individuals of European ancestry, respectively. The TC data was derived from a GWAS dataset conducted on 115 078 Europeans, which included metabolic biomarkers measured by Nightingale Health 2020 in the UK Biobank. The LDL data was derived from a GWAS dataset conducted on 201 678 UK Biobank European samples. The TG, HDL, apolipoprotein A-1 (Apo A-1), and apolipoprotein B (Apo B) data were obtained from a GWAS and MR analysis that was also performed on UK Biobank.24 The UK Biobank is a major collaborative research program that recruits and longitudinally tracks the health of 500 000 volunteers aged 40–69 years.25 The LGS data was derived from a GWAS meta-analysis of 256 523 Europeans aged 60 years and older from 22 cohorts.22
2.4 Instrumental variable selection
To fulfill assumption 1, the genetic variants closely associated with exposures were first selected (PIVs < 5E-08), and were then clustered within ±10 000 kilobase distance of the linkage disequilibrium threshold R2 < 0.001. To fulfill assumption 2, DM, CKD, AD and Cachexia were selected as confounders for Figure 1A, and basal metabolic rate (BMR) and malabsorption were selected as confounders for Figure 1B. Subsequently, the genetic variants with p values in the exposures greater than those in the confounders were eliminated from the final genetic variants obtained for assumption 1. To fulfill assumption 3, the genetic variants with p values in the exposures greater than those in the outcomes were eliminated on the basis of assumption 2.
In addition, the pleiotropic IVs were identified and removed using Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO).26 The F statistic was used to assess the strength of the selected IVs, and a value greater than 10 is usually considered powerful enough to mitigate potential bias.27 In this study, the F statistics of all IVs that were ultimately included were greater than 10. The F-statistics was computed as F = R2(N-2)/(1-R2), where N is the sample size of the GWAS studies and the R2 is proportion of the variability of exposure explained by each instrument.
2.5 Mendelian randomization analysis
In this study, the inverse variance weighted (IVW) method with multiplicative random effects was used as the primary method for estimating the causal relationship between exposures and outcomes. However, when the MR-Egger intercept p < 0.05, which indicates that the included IVs were conditioned with directed pleiotropy, the IVW results were considered not meaningful. The Cochran's Q test was used to estimate whether there was a high degree of heterogeneity among the included IVs.28 If the heterogeneity was not significant, the IVW fixed effects model would be used, otherwise a random effects approach would be applied instead. Since the Cochran's Q test for most of the outcomes in this study showed a relatively high level of heterogeneity, the IVW random effects approach was used by default. Moreover, the MR-Egger and weighted median methods were employed in this study as a complement to the IVW method.29 All MR analyses were performed using the R software package “TwoSampleMR” (https://github.com/MRCIEU/TwoSampleMR).
2.6 Sensitivity analysis
To ensure the reliability of the MR results, several sensitivity analyses were performed in this study. Prior to MR analysis, outliers were first identified and removed by the MR-PRESSO test.26 As mentioned earlier, the Cochran's Q test was used to examine the heterogeneity among the included IVs, with a p value <0.05 as the significance threshold.28 To test the horizontal pleiotropy, the MR-Egger intercept test was performed with a significance threshold of p value <0.05.
3 RESULTS
3.1 Effect of genetically predicted lipid profile on muscle atrophy
The heterogeneity test had no impact on the results because the random effects model was used for all IVW analyses in this study (see Figure 2, Tables S1 and S2 for the results). In the MR analysis with LGS as the outcome, IVW analysis showed no significant effects of TG, TC, HDL, LDL, Apo A-1 and Apo B on LGS (p > 0.05).
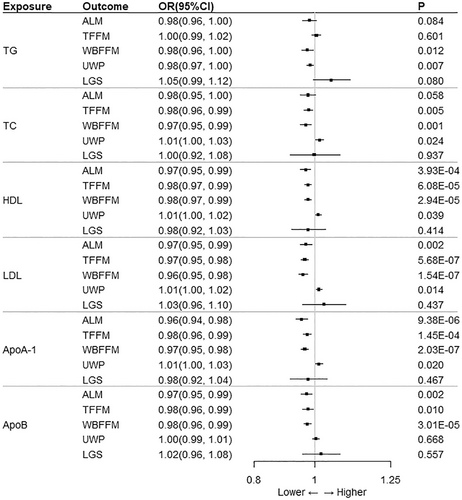
There was no significant causal relationship between TG and muscle atrophy. Specifically, IVW analysis showed no significant effect of TG on ALM and TFFM (p > 0.05). Although IVW analysis showed a p < 0.05 for the effect of TG on WBFFM and UWP, MR-Egger analysis indicated pleiotropy between these two causal pairs (intercept p < 0.05).
In addition, IVW analysis also showed that there was no significant correlation between TC and ALM (p > 0.05). TC had a significant effect on TFFM and WBFFM, both exhibiting a negative correlation (OR < 1; p < 0.05), and also had a significant effect on UWP, but with a positive correlation (OR > 1; p < 0.05). According to the intercept of MR-Egger regression, there was no horizontal pleiotropy between exposure and outcome (intercept p > 0.05). Besides, both the MR-Egger and Weighted median methods were in the same direction as the IVW analysis method.
In the MR analysis with HDL as the exposure, IVW analysis showed a correlation between HDL and TFFM, WBFFM, and UWP (p < 0.05). However, according to the intercept of MR-Egger regression, there was horizontal multidimensionality between exposure and outcome (intercept p < 0.05), and the IVW results were not significant. HDL had a significant effect only on ALM and the two parameters were significantly negatively correlated (OR < 1; p < 0.05).
IVW analysis showed that LDL was negatively associated with ALM, TFFM and WBFFM (OR < 1; p < 0.05), whereas LDL was positively associated with UWP (OR > 1; p < 0.05). There was no horizontal pleiotropy between exposure and outcome according to the intercept of MR-Egger regression (intercept p > 0.05). Both the MR-Egger and weighted median methods were in the same direction as the IVW analysis method.
The MR results with Apo A-1 as the exposure were consistent with HDL. The MR-Egger analysis of Apo A-1 for TFFM, WBFFM, and UWP showed horizontal pleiotropy between exposure and outcome (intercept p < 0.05), which was not significant for the IVW results. Similarly, Apo A-1 had a significant effect only on ALM and the two parameters were significantly negatively correlated (OR < 1; p < 0.05).
The MR-Egger analysis of the association between Apo B and ALM showed horizontal pleiotropy between exposure and outcome (intercept p < 0.05), whereas IVW analysis was not significant. IVW analysis showed a significant effect of Apo B on TFFM and WBFFM, both with a negative correlation (OR < 1; p < 0.05).
3.2 Effect of genetically predicted muscle mass on lipid profile
The heterogeneity test had no impact on the results because the random effects model was used for all IVW analyses in this study (see Figure 3, Tables S3 and S4 for the results). In the MR analysis with ALM as the exposure, IVW analysis showed no significant effect of ALM on HDL (p > 0.05). In contrast, ALM had a significant effect on TG, TC, LDL, Apo A-1, and Apo B with a negative correlation (OR < 1; p < 0.05). According to the MR-Egger test of pleiotropy, there was no pleiotropy between exposure and outcome (intercept p > 0.05). The MR-Egger and weighted median methods were in the same direction as the IVW analysis method (OR < 1).
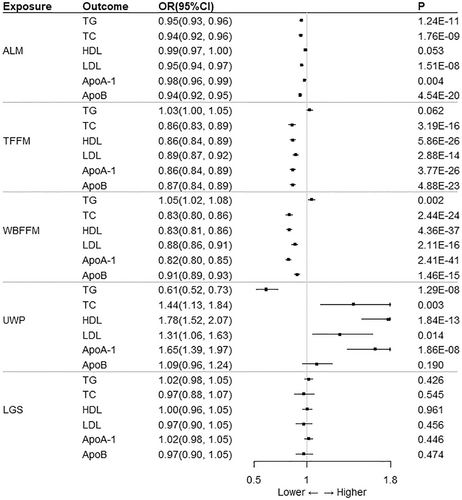
IVW analysis showed no significant effect of TFFM on TG (p > 0.05). There was pleiotropy in the MR-Egger test of pleiotropy of TFFM on HDL, Apo A-1 and Apo B (intercept p < 0.05), which made the IVW results invalid. TFFM had a significant effect only on TC and LDL, both exhibiting a negative correlation (OR < 1; p < 0.05; intercept p > 0.05). Similarly, the MR-Egger and weighted median methods were in the same direction as the IVW analysis method (OR < 1).
The MR-Egger test of pleiotropy indicated that WBFFM was pleiotropic for TG and HDL (intercept p < 0.05), so the IVW results were not significant. In addition, IVW analysis showed that WBFFM was significantly negatively correlated with TC, LDL, Apo A-1, and Apo B (OR < 1; p < 0.05).
IVW analysis also showed a significant negative correlation between UWP and TG (OR < 1; p < 0.05), and a significant positive correlation between UWP and TC, HDL, LDL, and Apo A-1 (OR > 1; p < 0.05). In contrast, LGS had no significant effect on TG, TC, HDL, LDL, Apo A-1, and Apo B (p > 0.05). According to the MR-Egger test of pleiotropy, LGS was not pleiotropic between exposure and outcome in the MR analysis with UWP and LGS as exposures (intercept p > 0.05).
4 DISCUSSION
This study demonstrated a bi-directional causal relationship between lipid profile and muscle atrophy-related characteristics through in-depth MR analysis using GWAS data. The results showed that the lipid profile predicted by genetic variants was negatively correlated with muscle mass and positively correlated with UWP, and was not causally related to LGS. On the other hand, the muscle mass predicted by genetic variants was negatively correlated with lipid profile; the UWP predicted by genetic variants was predominantly positively correlated with lipid profile; while the LGS predicted by genetic variants had no causal relationship with lipid profile. This suggests that high lipid profile may lead to muscle atrophy by exerting an effect on muscle mass, but has no significant effect on muscle strength. On the other hand, increased muscle mass may reduce the incidence of dyslipidemia. The present study is the first to demonstrate a bi-directional causal relationship between lipid profile and muscle atrophy-related characteristics through bi-directional MR analysis.
A previous observational study of 6288 adolescents reported an inverse causal relationship between ALM and HDL concentrations.30 The same conclusion was reached by another study, which showed that ALM in adolescents could be used to predict the risk of dyslipidemia.31 This is also consistent with the causal relationship between ALM and HDL obtained from our MR analysis. However, another observational study of 288 adult males reported that HDL significantly declined along with the reduction of muscle.32 The reason for such conflicting results may be attributed to inconsistencies between the included or the presence of multiple confounding factors. The last mentioned study also concluded that TG and TC were significantly elevated in patients with sarcopenia.32 This is consistent with the results obtained from our MR analysis showing that ALM has a significant negative correlation with TG and TC. Two other observational studies of 9477 adults over 40 years of age and 1336 minors over 12 years of age, respectively, indicated that an increase in TG-glucose index was significantly negatively correlated with ALM.33, 34 However, in our MR analysis, the causal effect of TG on ALM was not significant, which was speculated to be because of a confounding effect of glucose on ALM reduction.35 Further, it has been shown that muscle mass is not significantly correlated with LDL and TG concentrations.31, 36 In this study, we also found that the effects of TFFM and WBFFM on TG concentration were not significant, but there was a significant negative correlation between ALM and TG. This phenomenon may be due to the error generated by different muscle mass indicators, in addition to the effect of confounding factors.19, 20
To date, the association between walking speed and lipid profile has rarely been studied. A cross-sectional study including 5520 participants in Chile showed that faster UWP was associated with a better lipid status.7 One community-based observational study found that the faster the UWP, the higher the level of HDL concentration in older adults.37 This is consistent with the present study. In addition, this result is further supported by an RCT of 67 older women, which showed a significant negative correlation of UWP with TG and TC, but not with LDL.38 Another RCT of 68 participants also reached the same conclusion.39 In our study, the negative effect of UWP on TG was consistent with the two RCTs above. However, we found a significant positive correlation between UWP and TC and LDL, which may be attributed to confounding factors or potential pleiotropy. Thus, further investigations with different types of datasets are needed to clarify our findings.
GS has been recognized as an estimator of muscle strength and can be used to predict future disability, morbidity, and mortality.22 A previous cross-sectional study including 2677 adults showed that decreased GS was significantly associated with increased TG concentrations.40 Several recent observational studies have further demonstrated that decreased GS is also significantly associated with decreased HDL concentrations.41-45 However, an observational study of 16 149 Japanese adults reported that GS was negatively associated with dyslipidemia for all men and women under 50 years of age.46 In contrast, for women over 50 years of age, no significant association between GS and dyslipidemia was found.46 Another observational study also concluded that there was no significant correlation between GS and lipid profile in individuals over 65 years of age.47 The data of LGS used in our study was also obtained from elderly people above 60 years of age, and we also found that LGS was not associated with lipid profile. In addition to age, there were other confounding factors such as gender and ethnicity. The causal relationship between LGS and lipid profile needs to be further analyzed with different GWAS datasets.
This study is the first to investigate the causal relationship between lipid profile and muscle atrophy-related characteristics through MR analysis. We employed bi-directional two-sample MR analysis to overcome the potential bias of reverse causal relationship. Further, for the purpose of minimizing confounding factors, we excluded IVs representing some correlated diseases such as DM, CKD, and AD. In order to ensure the validity of IVs, we also excluded those IVs with p values in exposures greater than those in confounders and outcomes, and removed some outliers by MR-PRESSO. However, there are some limitations that cannot be avoided in this study. First, the GWAS data used in this paper were all derived from European ancestry, so our results may only apply to this particular population group. Second, muscle atrophy and lipid profile are known to vary by age and sex, but due to the use of pooled data, age- and sex-specific analyses were not possible. Third, although we made every effort to control the effect of confounders, it was not possible to exclude all confounders and the effect of confounders on the results still could not be ruled out. Finally, while we could provide genetic variants to analyze the potential causal relationship between lipid profile and muscle atrophy-related characteristics, we could not clarify the specific biological mechanisms, so the relationship still needs to be further investigated.
The findings of this MR analysis suggest that hyperlipidemia may affect muscle mass and lead to muscle atrophy, but has no significant effect on muscle strength. On the other hand, increased muscle mass may reduce the incidence of dyslipidemia. These findings unveil the possibility that we can use a patient's lipid profile to predict muscle mass and stabilize lipid levels to prevent muscle atrophy. In turn, we can also predict a patient's lipid profile from muscle mass. In clinical practice, we can increase muscle mass through nutritional supplementation and physical activity, which may help stabilize the lipid profile and thus reduce the incidence of cardiovascular disease.
AUTHOR CONTRIBUTIONS
Zhenhan Deng, Liangjun Li and Kun Chen conceived and designed the study. Kun Chen, Peng Gao and Xiaoxiang Fang collected data. Kun Chen, Kexing Tang, Pan Ouyang and Zongchao Li analyzed data. Kun Chen, Peng Gao, Xiaoxiang Fang and Kexing Tang wrote the first draft of the manuscript. All authors revised the manuscript and gave final approval to the submitted versions.
ACKNOWLEDGMENTS
We thank the UK Biobank and the MRC Integrated Epidemiology Unit for providing the open GWAS database.
FUNDING INFORMATION
Guangdong Basic and Applied Basic Research Foundation 2021A1515220030 (Z.D.), Natural Science Foundation of Hunan Province number 2020JJ8043 (L.L.), the Key project of Hunan Provincial Health Commission number 20201902 (L.L.), the Project of Hunan Provincial Health Commission number c2019133 (L.L.) and the Hunan Provincial Clinical Medical Technology Innovation Guiding Project number 2020SK53307 (L.L.).
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
Our study did not require further ethics committee approval as it used publicly available GWAS summary statistics. No new data were collected, and no new ethical approval was required.




