Twenty-Four-Month Outcomes Following Temperature-Controlled Radiofrequency Treatment for Septal Swell Body Hypertrophy: An Open-Label, Single-Arm Multicenter Study
Funding: This study was funded by Aerin Medical.
1 Introduction
Summay
- TCRF treatment of the SSB resulted in long-term improvements in NAO symptoms through 24 months.
- A significant proportion of patients were treatment responders through 24 months.
- No new device- or procedure-related adverse events were reported in the current follow-up period.
The septal swell body (SSB) is a fusiform structure composed of vasoerectile tissue located on the septum, anterior to the middle turbinate and within the nasal airflow path. It plays a similar role to the inferior turbinates, affecting airflow and contributing to resistance at the nasal valve [1]. Although multiple factors contribute to nasal obstruction (NAO), SSB hypertrophy is often overlooked as a cause of breathing difficulty. Despite its potential to affect nasal airflow, the SSB's role in NAO has historically received little attention. Recent studies highlight its impact on nasal airflow, particularly its relationship with the nasal valve and its vasoerectile nature [2-4]. Temperature-controlled radiofrequency (TCRF) treatment of the SSB has emerged as a minimally invasive NAO treatment option [5]. The 3- and 12-month outcomes of this study demonstrated significant improvements in SSB size and NAO symptoms, with high responder rates and sustained relief across patient-reported outcomes such as the nasal obstruction symptom evaluation (NOSE) scale and numeric rating scale (NRS) ease of breathing [6, 7]. This paper presents the long-term follow-up results of the study, evaluating TCRF's durability in treating the SSB for NAO.
2 Methods
This study reports 24-month results as a follow-up to an earlier multicenter, open-label TCRF study on severe NAO from SSB hypertrophy [6, 7]. The TCRF device, comprised of the VivAer Stylus (Aerin Medical, Mountain View, CA), was used with the Aerin Console. During the procedure, local anesthesia was administered, followed by bilateral TCRF treatment targeting the SSB. The treatment was performed at 60°C, 4 W, with 18 s of active treatment and 12 s of cooling. Treatment of other nasal structures (e.g., nasal valve area and inferior turbinate) was not allowed.
Eligible participants were adults with a baseline NOSE score of ≥55, and SSB hypertrophy presents limiting middle turbinate visualization and symptomatic relief observed after SSB decongestion [6, 7]. Exclusion criteria included recent nasal surgery (within 6 months) and other structural abnormalities like severe septal deviation or turbinate hypertrophy deemed the primary NAO cause. (See Table S1 for full inclusion/exclusion criteria.)
Outcome measures included NOSE scores, NRS ease of breathing, and the 22-item Sino-Nasal Outcome Test (SNOT-22) scores that are analyzed against baseline. Data analysis followed 12-month analysis methods [7]. In brief, paired t-tests compared baseline and follow-up scores. Responder analysis was defined as a ≥20% NOSE score reduction (or improvement in 1 severity category) from baseline. Demographic data were summarized with means and percentages. Adverse events (AEs) were tracked for 24 months via telephone follow-up, evaluating symptom changes, medication use, and NAO-related interventions. Patients undergoing additional nasal procedures were excluded from analysis.
3 Results
The study enrolled 70 participants, with clinical characteristics as previously described [6]. Of these, 58 completed the 24-month follow-up.
3.1 Outcome Measures at 24 Months
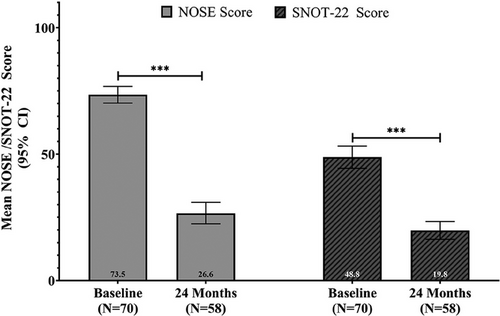
At 24 months, sustained improvements were observed across all outcome measures (Table 1). The treatment responder rate was 78.6% at 24 months, slightly lower than the 84.3% at 12 months. The mean NOSE score decreased from 73.5 at baseline to 26.6 at 24 months (95% CI 22.4–30.9), indicating a 63.5% adjusted mean improvement (p < 0.001) (Figure 1). SNOT-22 scores decreased from 48.8 to an adjusted mean of 19.8 (95% CI 16.3–23.4), marking a 58.0% improvement at 24 months (p < 0.001) (Figure 1). The mean NRS ease of breathing score decreased from 6.4 at baseline to 2.2 at 24 months (95% CI 1.7–2.7), indicating a 65.6% mean improvement (p < 0.001).
| Variable | Baseline | 12 Months | 24 Months | Mean change (baseline to 24 months) | % Change (baseline to 24 months) | p value* |
|---|---|---|---|---|---|---|
| NOSE score |
73.5 (70.2, 76.8) N = 70 |
25.1 (20.9, 29.2) N = 62 |
26.6 (22.4, 30.9) N = 58 |
−46.6 (−50.9, −42.4) |
−63.5 % (−69.1%, 57.9%) |
< 0.001 |
| Ease of breathing (NRS) |
6.4 (6, 6.8) N = 70 |
2.4 (1.8, 2.9) N = 62 |
2.2 (1.7, 2.7) N = 58 |
−4.2 (−3.6, −4.8) |
−65.6% (−55.5%, −75.8%) |
< 0.001 |
| SNOT-22 total symptom scores |
48.8 (44.4, 53.2) N = 70 |
18.7 (15.3, 22.2) N = 62 |
19.8 (16.3, 23.4) N = 58 |
−28.9 (−32.4, −25.3) |
−58.0% (−64.7%, −51.2%) |
< 0.001 |
| % Treatment responders (NOSE) | — |
84.3% (−6.8, 52.2) N = 59 |
78.6% (−12.7, 62.9) N = 55 |
— | — | — |
- Note: Mean changes in NOSE score, NRS ease of breathing, and SNOT-22 scores overall and domain scores from baseline to 12 and 24 months following temperature-controlled radiofrequency treatment for septal swell body hypertrophy are presented as follows: Baseline scores are shown as mean (95% CI), while 12- and 24-month values are reported as least squares mean (LS mean) with 95% confidence intervals (CI). p Value is for LS mean change from baseline. Treatment responders were the proportion of subjects who achieved ≥20% improvement (or improvement in 1 severity category) from baseline NOSE score, n (%) (95% CI).
- Abbreviations: CI, confidence interval; LS, least squared; NOSE, nasal obstruction symptom evaluation; NRS, Numeric Rating Scale; SD, standard deviation; SNOT-22, 22-item Sino-Nasal Outcome Test.
3.2 Adverse Events
No device- or procedure-related AEs were reported between 12 and 24 months, consistent with the 3- to 12-month safety results [6, 7]. During the first 3-month study interval, 12.9% of participants reported AEs at least potentially related to the device or procedure (Table 2).
| Number of events reported (onset) | Relatedness | |||||
|---|---|---|---|---|---|---|
| Adverse event | 0–3 Months | 3–12 Months | 12–24 Months | Device | Procedure | Severity |
| Visual acuity change |
1 (Study Day 0) |
0 | 0 | No | Definitely | Mild |
| Purulent nasal discharge |
1 (Study Day 0) |
0 | 0 | Possibly | Probably | Mild |
| Headache |
1 (Study Day 0) |
0 | 0 | No | Possibly | Mild |
| Nasal scarring | 0 |
1 (Study Day 96) |
0 | No | Possibly | Mild |
| Epistaxis | 0 |
1 (Study Day 32) |
0 | No | Probably | Moderate |
|
Nasal congestion/ obstruction |
1 (Study Day 0) |
0 | 0 | No | Probably | Moderate |
| Nasal scarring | 0 |
1 (Study Day 167) |
0 | Definitely | Definitely | Moderate |
| Sinusitis |
1 (Study Day 7) |
0 | 0 | No | Possibly | Moderate |
| Vasovagal reaction |
1 (Study Day 0) |
0 | 0 | No | Probably | Mild |
- Note: Study Day 0 is day of study procedure.
- Abbreviation: AE, adverse event.
4 Discussion
These 24-month results affirm the long-term efficacy of TCRF treatment for SSB hypertrophy. Significant improvements in NOSE and SNOT-22 scores, and NRS ease of breathing scores were sustainably maintained over time. These findings align with earlier 3- and 12-month results, highlighting durable symptom relief. The treatment offers enduring improvements for most patients, shown by substantial NOSE score reductions and robust treatment responder rates.
In terms of safety, the absence of AEs from 12 to 24 months strengthens TCRF's well-documented safety profile. TCRF may be considered safer than traditional RF, as it is limited to a peak temperature of 60°C. This controlled thermal minimizes tissue damage while ensuring optimal mucosal healing, achieved through the generation of consistent lesion depths (up to 5 mm) [8].
In contrast, traditional RF may not offer the same level of precision in thermal regulation, increasing the risk of complications such as char formation, scarring, and toughening of the mucosa, as well as more severe side effects like necrosis [9]. Moreover, conventional RF is primarily utilized for structural issues like turbinate hypertrophy, whereas TCRF applications extend to neurogenic conditions, such as chronic rhinitis, by targeting specific neural pathways located approximately 3–5 mm below the mucosal surface [8]. This precise targeting minimizes damage to surrounding tissue while effectively disrupting aberrant neural activity. Other techniques, like submucosal microdebriders, are more invasive, requiring operating room settings, and may pose a greater risk of bleeding. In contrast, TCRF preserves the integrity of the mucosa and underlying tissue and can be safely performed in-office.
This study provides compelling evidence that TCRF offers statistically significant, clinically relevant improvements that are sustained over a 24 month period, representing an effective solution for NAO from SSB hypertrophy. These results, together with earlier studies [6, 7], highlight the efficacy of TCRF treatment of SSB hypertrophy, a significant but often overlooked target for NAO. This study showed durable reductions in SSB-related NAO symptoms through 2 years. In addition to reducing obstructive tissue through shrinkage, TCRF may also ablate peripheral nerves in the treated area resulting in less reactivity of the tissue which may contribute to the longer term improvements observed in this study similar to what has been observed in TCRF targeting nerve hyperreactivity in chronic rhinitis [10]. Further anatomic and histologic study describing innervation of the SSB could be helpful to determine if the durable effect of TCRF ablation of the SSB could be attributed to destruction of nerve tissue as well as reduction of SSB tissue. In conclusion, the findings strongly support inclusion of TCRF in comprehensive NAO management protocols.
Acknowledgments
The authors thank Mark Hensley (Hensley Biostats) for statistical analysis and Natalie DeWitt (Accendo Scientific) for assistance with manuscript writing and preparation; both are independent consultants to Aerin Medical.
Conflicts of Interest
Jordan Pritikin: Consultant for Aerin Medical, Optinose, and Carelon. Stacey Silvers: Consultant for Aerin Medical and 3-D Matrix. Anthony Del Signore: Consultant for Neurent. Rakesh K. Chandra: Consultant for Regeneron, Optinose, Olympus, and Lyra Therapeutics. The remaining authors declare no conflicts of interest.




