Technique for ultrasound-guided radiofrequency denervation of genicular nerves for chronic knee pain
Abstract
Genicular nerve radiofrequency denervation (GNRFD), also called radiofrequency ablation or genicular neurotomy, has been demonstrated to be a safe and effective method of treating and managing chronic knee pain caused by osteoarthritis (OA). The genicular nerves have been identified as key sensory nerves that innervate the knee joint. Using ultrasound guidance, the genicular nerves can be heated to temperatures up to 80°C, creating a local neuronal lesion, causing denervation and therefore temporarily alleviating knee pain. GNRFD is an effective treatment for those with chronic knee pain in whom conservative treatment has failed, who are poor candidates for surgery or who are on an extended waiting list for their surgery. This article outlines the technique for introducing the radiofrequency (RF) probes so the tip is in close proximity to the genicular nerves using ultrasound guidance.
Introduction
According to data from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR 2019), 65,266 knee replacement surgeries were performed in Australia in 2018.1
Between 2005 and 2018, there was a 38% increase in total knee replacement (TKR), from 133 to 184 per 100,000 population.1 In 2015–2016, osteoarthritis (OA) cost the Australian health system an estimated $3.5 billion.1 Patients with symptomatic arthritis include night pain and pain with walking. Plain x-rays will show osteophytosis, joint space narrowing and an effusion. Anxiety, depression and psychological distress are commonly experienced in people with OA as they endure chronic pain and physical disability with increased opioid use and often poor treatment outcomes.1 There is no cure for OA, and the disease is long term and progressive. Treatment for OA aims to manage symptoms, increase mobility and improve quality of life. Current treatment consists of increasing activity, reducing body mass index (BMI), administration of painkillers and oral nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroid injections or viscosupplement injections and eventually arthroscopy and TKR.1
It has been shown that corticosteroid injections (CSI) supplements and arthroscopy are not effective treatments for managing knee OA.2
In patients who have had failed conservative treatment or failed surgery or who are poor candidates for surgery, genicular nerve radiofrequency denervation (GNRFD) has been found to offer temporary relief of pain lasting up to 6–12 months.2
Knee pain is generated from pain signals from the genicular nerves; therefore, denaturing the proteins in the nerve sheath using heat produced by high frequency radio waves, the pain transmission is ‘switched off’.2 Pain relief is temporary as nerves do regenerate, and therefore, the pain will return.2
GNRFD has generally been performed using fluoroscopic guidance; however, there are contraindications with this method. The nerve, artery and soft tissue structures are not visualised with fluoroscopy. This technique does not account for any anatomical variance and relies on the nerve being at a certain position above the femoral epicondyles and below the tibial epicondyle. If the probe placement is not accurately positioned adjacent to the nerve, denervation will not occur and therefore the procedure will fail. If the radio frequency (RF) probe pierces the genicular artery, complications of pseudoaneurysm, hemarthrosis, haematoma and arteriovenous fistulas can occur.3 There is also the issue of using ionising radiation.
Using ultrasound guidance, we have found that in most cases the genicular arteries and nerves can be visualised. This allows for accurate placement and visualisation of the RF probe over the nerve and away from the artery during the entire procedure, thereby reducing potential complications.
Anatomy
The genicular nerves are sensory branches of the tibial, common peroneal and obturator nerves. They provide innervation to the capsule of the knee joint and intra-articular and extra-articular ligaments.4
The genicular nerves comprise the superior medial genicular nerve (SMGN), superior lateral genicular nerve (SLGN), inferior medial genicular nerve (IMGN), inferior lateral genicular nerve (ILGN) and middle and recurrent tibial genicular nerve.5
The nerves we routinely target for RFD using ultrasound guidance are the SMGN, SLGN and IMGN (Figure 1). Occasionally, but rarely ILGN is targeted, however its close proximity to the common peroneal nerve can be associated with increased risk of foot drop.6
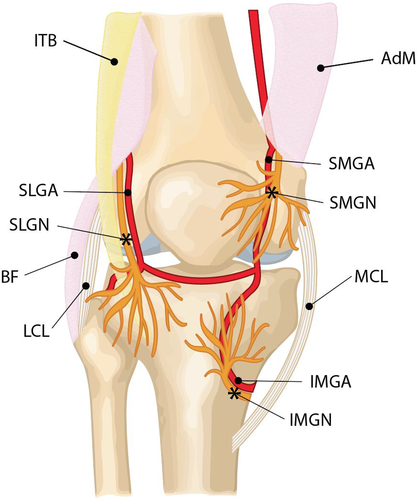
The genicular nerves are tiny and difficult to visualise on ultrasound; however, it is assumed they run with the genicular arteries. The close proximity of the nerves with the genicular arteries provides a useful landmark for targeting the nerves under ultrasound. While the genicular nerves and arteries have a variable course proximally, it was shown in cadaveric studies that they all had a constant distal contact with the femur and tibia, closely adjacent to the periosteum.3
The SLGN originates from the common peroneal division of the sciatic nerve 8–10 cm superior to the joint line of the knee. It travels towards the superolateral aspect of the knee capsule deep to the biceps femoris muscle and the iliotibial band.3 The SLGN and associated superior lateral genicular artery (SLGA) pass close to the junction of the lateral epicondyle and distal shaft of the femur (Figure 1). Han et al. performed a study in 2018, calculating the distance of the SLGN to the bony cortex and estimated it ranged from 3.8 to 5.7 mm.5
The SLGN innervates the lateral collateral ligament and the lateral and anteroinferior region of the capsule.5
The SMGN and IMGN are branches of the tibial nerve in the popliteal fossa. The tibial nerve is a terminal branch of the sciatic nerve. It runs medially through adipose tissue into the deep fascia and then towards the midpoint between both heads of gastrocnemius muscle. There are three branches of the tibial nerve which join the superior, middle and inferior medial genicular arteries.3
The SMGN and superior medial genicular artery (SMGA) course around the femoral shaft and pass between the adductor magnus tendon and the femoral medial epicondyle and then descend anterior to the adductor tubercle4 (Figure 1).
The SMGN runs along the medial edge of the femoral condyle 2.1–3.9 mm from the bony cortex.5
The IMGN and inferior medial genicular artery (IMGA) are situated horizontally around the lower part of the medial tibial epicondyle and pass underneath the medial collateral ligament. They can be seen midway between the peak of the tibial medial epicondyle and the distal fibres of the medial collateral ligament (MCL) as it inserts onto the tibia4 (Figure 1). The IMGN can be seen 1.1–2.9 mm from the bony cortex of the tibial medial epicondyle.5
The SMGN and IMGN innervate the posterior and perimeniscal portion of the capsule and the synovial lining. The IMGN surrounds the cruciate ligaments and is extra synovial.4
Radiofrequency Denervation
Radiofrequency denervation (RFD), also called radiofrequency ablation, neurotomy or cooled radiofrequency therapy, is a procedure where the nerve sheath cells are destroyed using heat produced by high frequency radiowaves.7 There are three different types of radiofrequency denervation procedures – conventional, pulsed and cooled.7
Conventional RFD applies a high heat – up to 80°C – via a probe that emits an intense alternating electrical field over a period of 60–90 s. This thermal degradation of the nerve causes denaturing of the proteins in the targeted tissue.2, 8
Pulsed RFD uses short bursts of radiofrequency energy (10–20 milliseconds) separated by long silent phases (480 milliseconds). The surrounding tissue temperature is maintained at 42°C.3, 9-11 The effects of pulsed RFD are more reversible and less destructive and so therefore do not have the lasting effect of pain relief provided by conventional or cooled RFD when applied to genicular nerves.10
Cooled radiofrequency ablation (CRFA) circulates water within the probe which carries heat away from the tissue–tip interface, thus reducing burning of the surrounding tissue while delivering more energy.8 The probe temperature is set to 60°C but manages to increase the temperature of the surrounding tissues to 80°C. This effect of being able to deliver more energy with less probe heat creates a larger lesion and more damage to the nerve.8 This larger lesion size may increase the amount of time the patient has pain relief as the nerve takes longer to regenerate.
Technique
Patients typically present with a referral from their GP or specialist for GNRFD. All patients then have a consultation with the radiologist who clinically examines them and assesses their likelihood to be a potential candidate for GNRFD.
Before progressing to GNRFD, patients undergo two nerve blocks where long acting local anaesthetic, lasting up to 6 hours is administered to each of the nerves. The nerve blocks are performed using ultrasound guidance and are separated by at least 48 hours to one week. Our department protocol is to use 0.5–1 ml of dexamethasone with 1.5–2 ml of bupivacaine. The volumes are not as critical as they are with other modalities, as by using ultrasound we are able to visualise the injectate tracking along the nerve sheath.
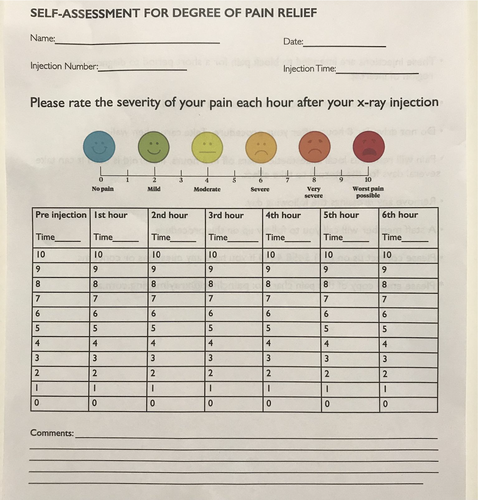
The patients are given a pain chart (see Appendix A) to fill out prior to the injections to ascertain their current pain level. The patient then documents their pain score each hour on the hour for six hours after their nerve block. Each patient must demonstrate significant pain relief following these nerve block trials. The aim is for complete pain relief, but if the pain reduces by 50% then this is significant for the patient and is considered a positive trial.
- A reduction in the severity of pain of at least 50% according to the pain chart numbers over the six hours;
- Patient’s own opinion as to whether the block was successful;
- The degree of muscle tone, obesity and the incentive of the patient to help themselves are taken into consideration;
- The radiologist’s opinion, taking all the above variables into consideration. Each patient is assessed on merit.
Once a consensus has been reached, the patient proceeds to GNRFD.
Patients are excluded if they have a pacemaker in situ based on the inability to have a cardiologist or electrophysiologist onsite to monitor the patient. From a health standpoint, it has been shown that pacemakers are not a contraindication for performing GNRFDs.11
Equipment
A Canon series Aplio 550 machine was used with either a 18L7 MHz, 14L5MHz or a 10C3 MHz transducer depending on body habitus with the 14L5 MHz being the most commonly used. The procedures were performed using Conventional GNRFD with the Pain Management Generator, version 4, advanced, software version V4.1.1A with the Diros Technology INC Radiofrequency probe, the OWL 18 gauge Sharp Curved RF Insulated Cannula, which has an activated tip length of 10mm, and the MACROLYTE Dispersive Electrode grounding pad.
A strict aseptic technique was used with all injections. Sterility is paramount to avoid infection and localised inflammation. The following sterile materials included; a dressing pack, 3-ml, 5-ml and 10-ml syringes, needles (drawing-up – 18 gauge (G), 25G × 38 mm, 22G × 50 mm, 22G × 70 mm), chlorhexidine in isopropyl alcohol 70%, surgical gown, gloves, surgical drapes and dressings.
Both short- and long-acting local anaesthetics were used, 1% lignocaine and 5% bupivacaine to anaesthetise the surrounding soft tissues.
Preparation
- The patient is semi-reclined or supine on a clinic bed, to prevent a vaso vagal response, with the affected leg exposed from the mid-thigh down.
- The affected knee is supported by a triangular wedge in order to position the knee at approximately 30 degrees of flexion (adjusting according to patient's comfort).
- A surgical drape is placed over the knee to prevent cross-contamination.
- If there is a large joint effusion present in the suprapatellar recess, the effusion is aspirated prior to the blocks and the GNRFD. Documentation of the aspiration is important as it can be a confounding factor in the success of the procedures and therefore must be taken into consideration when assessing the patients’ results.
Ultrasound Techniques for Isolating each of the Genicular nerves
We do not have a dedicated order in which the nerves are blocked.
Superior Lateral Genicular Nerve
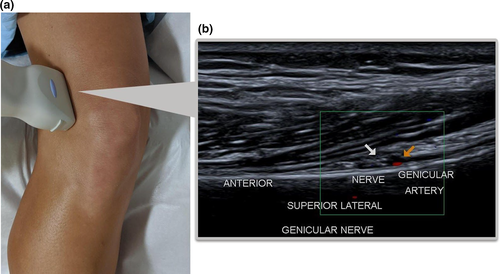
- The transducer is placed coronally over the lateral epicondyle and moved slowly in a cephalic direction parallel to the femur following the curve of the lateral femoral shaft until the cross-section of the SLGA is visualised. Colour Doppler is useful to visualise the artery and, if seen, the adjacent SLGN deep to the Iliotibial band (ITB) and biceps femoris muscle (Figure 2).
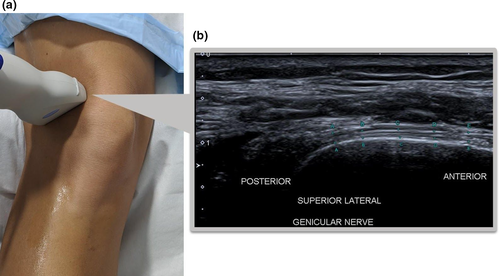
- The transducer is rotated approximately 90° to visualise the SLGN fibres in a longitudinal orientation, coursing posterior to anterior, adjacent to the femoral shaft (Figure 3).
Superior Medial Genicular Nerve
- The transducer is placed coronally over the medial epicondyle and moved slowly in a cephalic direction following the femoral shaft until the cross-section of the SLGA is visualised with colour Doppler and, if seen, the adjacent SMGN located deep to the Adductor magnus muscle (Figure 4).
- The transducer is rotated approximately 90 degrees to visualise the SMGN fibres in a longitudinal orientation, coursing posterior to anterior, adjacent to the femoral shaft (Figure 5).
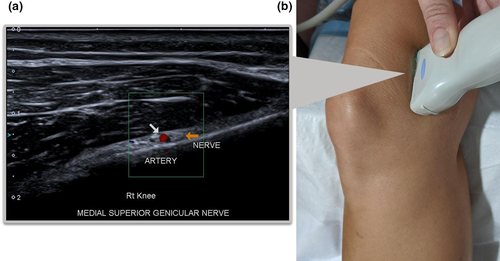
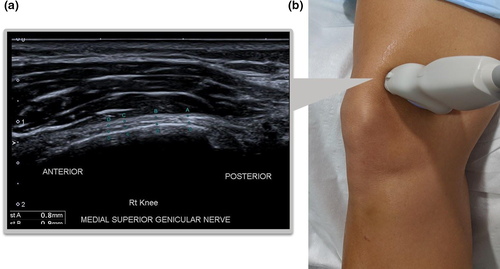
Inferior Medial Genicular Nerve
- The transducer is placed (for most patients the 18L7 MHz was used due to its superficial location) in a coronal orientation over the MCL at the level of the medial meniscus. The transducer is moved inferiorly following the MCL to its attachment on the medial condyle of the tibia until the IMGA is seen on colour Doppler with the adjacent IMGN (Figure 6).
- The transducer is rotated until the nerve fibres can be seen in a longitudinal orientation curving around the tibial condyle (Figure 7).
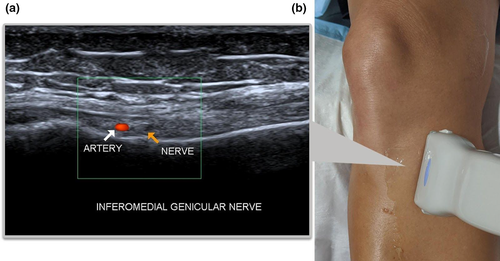
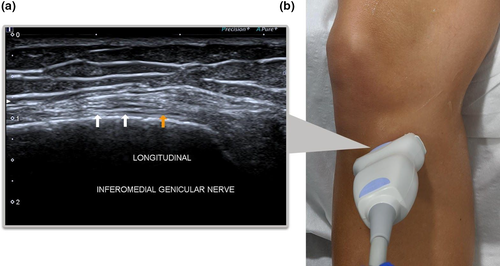
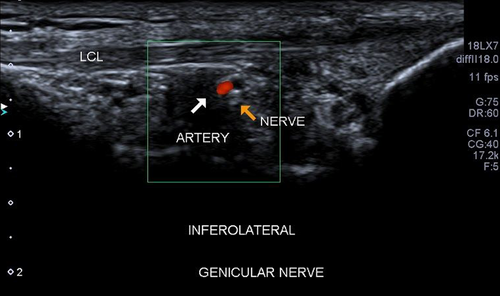
Inferior Lateral Genicular Nerve
Although it is not standard practice to perform GNRFD on the ILGN due to the apparent risk of causing foot drop6 and has the least innervation of the knee, we thought it was worth a brief mention. The transducer is placed parallel to the lateral collateral ligament (LCL) of the knee. The ILGA and the ILGN can be seen deep to the mid-distal segment of the ligament (Figure 8).
GNRFD Protocol
- The patient is consented and confirmed they have a driver to get home.
- The grounding pad is secured to the patient.
- The nerves are isolated with ultrasound and their skin marked to aid in quick relocation.
- Under ultrasound guidance, the patient's skin and soft tissues are anaesthetised down to the periosteum with lignocaine 1% using a 25-gauge needle initially, changing to a longer, 22 gauge only if needed.
- The needle is replaced with the sharp curved RF cannula and the nerve flooded with bupivacaine 5%. It is important to pre-fill the cannula with bupivacaine prior to inserting, as introducing air at this point will cause a ‘dirty shadowing’ artefact obscuring visualisation of the nerves.
- While holding the cannula enlocated, the RF probe is inserted.
- Utilising ultrasound, the active tip of the cannula is positioned parallel to the nerve. The cannula is manoeuvred until the longest segment of the GN is in contact with the active 10mm tip.
- For the larger patient where ultrasound cannot resolve the nerve as a separate entity to the GAs, the cannula can be guided adjacent to the GA in cross-section.
- The RFD is set to a temperature of 80°C for 120 s.
- The patient's tolerance during the procedure is monitored and if requested, the RFD is stopped, administering more lignocaine 1% perineural as needed, and then the procedure resumed.
The patient is contacted after 3 weeks, 3, 6 and 12 months to see whether their pain levels have decreased and their quality of life has been improved.
Conclusion
Most papers describing GNRFD technique were performed under fluoroscopic guidance. This technique is far from accurate and has associated potential risks to the genicular arteries. Using high frequency linear transducers and a sound knowledge of the anatomy, the genicular neurovascular bundle can be more reliably visualised using ultrasound, allowing more accurate placement of the RF probe.
With the COVID-19 pandemic cancelling elective surgeries, we have seen waiting times for total knee replacement surgery for some of our patients extended up to 2 years in our region. This has left many of our patients suffering physical, emotional and financial stress as they try to cope with crippling pain and immobility.
As we have demonstrated, ultrasound is an inexpensive, safe and accessible modality to use as guidance for GNRFD. This is a procedure which is well tolerated and can be repeated when pain returns.
Acknowledgements
We would like to thank Dr Gus Ferguson, Dr Richard Langford and Shaun O’Rourke for their review and commentary on the initial draft of this article. We would also like to thank Benjamin Dickens for his original graphic designs used in this article.
Authorship statement
We acknowledge that the authorship listing conforms with the journal’s authorship policy, and that all authors are in agreement with the content of the submitted manuscript.
Funding
No funding was received for this paper.
Disclosure
This paper is intended as an instructional guideline only. Because ultrasound guidance GNRFD is a relatively new procedure, we are still in the process of compiling clinical outcomes. At present, we do not have a statistically significant amount of data to give an inference on patients’ long-term results compared with other modalities. We look forward to sharing these data at a future date.
Ethical Approval
All people gave their informed consent prior to their inclusion in the study.
Author Contributions
Elizabeth Anne Merrin: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing-original draft (equal); Writing-review & editing (equal). Le-Anne Marise Grimshaw: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing-original draft (equal); Writing-review & editing (equal).