Dietary Variation Among Herculaneum's Victims of Mt. Vesuvius via Dental Microwear Texture Analysis
Funding: This project was funded by a grant from the National Science Foundation (NSF) (BCS 0922930).
ABSTRACT
The Roman site of Herculaneum provides an extraordinary opportunity to reconstruct ancient diets in the context of life course theory because everyone died simultaneously due to the eruption of Mount Vesuvius in AD 79. Objectives: The current study addresses three primary hypotheses: (1) Subadult diets changed as children aged, (2) adult diets differed from subadult diets, and (3) male and female diets differed as they age. Materials and Methods: This study includes dental microwear texture data from 58 adults (age 16+) and 23 subadults (age 0–16) recovered by Sarah Bisel from within and near to storage rooms adjacent to Herculaneum's beach. The adults include 27 females and 31 males. Dental microwear texture analysis (DMTA) employed standard procedures: data collection used a white-light confocal profiler at 100×. The DMTA variables were complexity, anisotropy, textural fill volume, and scale of maximum complexity. Statistical methods used Bayesian versions of analysis of variance and correlation (Bayes factors above 1.5 were considered meaningful and above 3.0 significant) as well as discriminant function analysis and binary logistic regression. Results: No differences emerged among the children. Adult diets were significantly lower than the subadults for anisotropy. Among the adults, age affected the females more, particularly for anisotropy. Discussion: Subadult diets did not vary by age, but they did vary. The lower adult anisotropy indicates each adult ate a greater variety of foods compared to the subadults. As females aged, however, their diets became more restricted compared to the males. Overall, age and sex affected the Herculaneum diet.
Ancient cemeteries often include people who died over long spans of time, perhaps hundreds of years or more. In these cemeteries dietary reconstructions are challenging because, without precise dates for each person, changes in dietary preferences over time can be confused with dietary variation at specific points in time. The Roman site of Herculaneum in Italy, however, produced hundreds of people who died simultaneously in a single mass casualty event. Herculaneum, therefore, provides a remarkable opportunity to focus on synchronic dietary variation within a population that perished at the same moment.
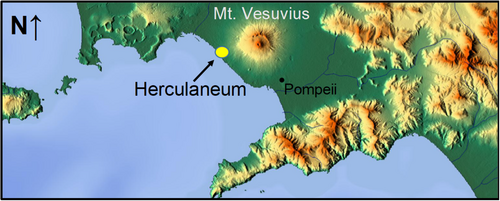
Herculaneum was a Roman port city, located west of Mount Vesuvius and north of Pompeii (Figure 1). The eruption of Mount Vesuvius in AD 79 is traditionally thought to have occurred in late August, but recent work indicates the eruption took place between October 24th and November 1st (D'oronzo et al. 2022); it ejected material high into the atmosphere that, at first, drifted southeast toward the city of Pompeii. Eventually, airborne ash and rock lost its buoyancy and returned to earth in the form of a pyroclastic surge that buried Herculaneum under 20 m of ash, sediment, and rubble (Capasso 2001; Wallace-Hadrill 2011). Famously, Pliny the Younger described the eruption plume as a great vertical shaft that spread out as it reached its maximum height; to him the plume had the shape of an umbrella pine, which has its branches concentrated at the top of a tall narrow trunk (Walsh 2006). Pliny's description, along with the remaining geological evidence today, makes it clear the eruption was a powerful event that produced several deadly phenomena including heavy ash falls, volcanic debris, and hot and fast-moving gasses.
1 The Victims at Herculaneum
Unlike the people of Pompeii who ended up entombed in cement-like layers of pumice, most of the people of Herculaneum died huddled in storage rooms near the shoreline. Their deaths resulted from exposure to temperatures exceeding 450°C, and a surge force that reached approximately 130 km per hour (Kent et al. 1981; Bisel 1987; Capasso 2001). The surge killed the people quickly, but skeletonization was not instantaneous (Oakley 2014; Schmidt et al. 2015).
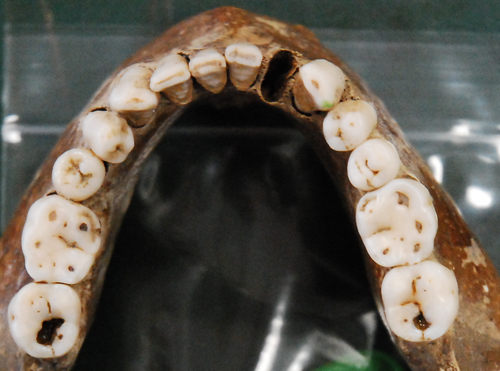
In the early 1980s, the National Geographic Society learned of the accidental unearthing of human remains near the old Herculaneum shoreline; the society hired osteologist Sara Bisel to oversee the subsequent excavation. Eventually, her crew recovered 154 well-preserved skeletons (Bisel 1991). Perhaps surprisingly, thermal affects to the skeletons were not extreme, and most bones and teeth lacked major heat-related damage (Figure 2). Some skeletal elements, primarily among the subadults, had calcined bones, but most were mottled to blackened, indicating they were protected from the worst of the heat by soft tissues (Schmidt et al. 2015). Additionally, dark organic and orange stains occurred periodically, particularly on the skulls. The orange staining consisted largely of iron but was not blood (Schmidt et al. 2017). Except for a few fractured teeth and some covered in desiccated soft tissue, most teeth survived the pyroclastic event in excellent condition.
In addition to Bisel's initial studies, Luigi Capasso independently conducted a detailed osteological analysis of the Herculaneum remains (Capasso 2001); today, the University Museum in Chieti, Italy houses the remains Bisel excavated. The current study refers to the remains excavated and studied by Bisel and Capasso as the Bisel/Capasso Herculaneum remains. There exists another collection of Herculaneum remains that were excavated in the 1990s after the Bisel-led excavation. They are not part of the current study, but publications based on the non-Bisel/Capasso Herculaneum skeletons are discussed and cited.
Herculaneum had a population around 5000 people, most of whom were enslaved people or freed slaves (see De Ligt and Garnsey 2012). It also was home to people holding a wide array of occupations including bakers, merchants, farmers, smiths, and soldiers. Sizable villas, many of which still stand, indicate wealthy people lived there and historical accounts state that Herculaneum was a popular spot to vacation (De Vos 1982; Maiuri 1998). The precise number of people who vacated the city prior to the eruption is unknown; it is also unclear why some people were not yet rescued at the time of the surge.
2 Roman Subsistence and Diet
Subsistence refers to the activities, tools, and natural resources (including plants and animals) exploited to meet the nutritional demands of a population (Hillson 1979). Historians report Romans subsisted on cereal grains, wine, olives, and dry legumes (see Prowse et al. 2004; Murphy, Thompson, and Fuller 2013; Rowan, Flohr, and Wilson 2017). They also collected wild foods from woodlands and pasturelands including herbs, berries, fruit, cucurbits, and root vegetables like radishes and leeks (e.g., Borgongino 2006). Some of the wild foods were cultivated after being collected; they were planted in gardens and likely served both nutritional and medicinal needs (Frayn 1975, p 33).
Traditionally, descriptions of Roman subsistence emphasize a marked dependence on the agricultural triad of cereals, olives, and grapes. Recent work, however, has reassessed the importance of animal husbandry, and archaeological evidence points to animal exploitation across the empire (Witcher 2016). Farmed animals included pigs, sheep, goats, and cattle (e.g., MacKinnon 2004; Erdkamp and Holleran 2019).
Roman exploitation of fish varied by region. There is more evidence for a sizable fishing industry in Italy, for example, than in regions like Spain. In Italy fishing and fish consumption is indicated by artwork, literary references, and archaeological remains, including amphorae used to store salted fish. There are mosaics in Italy and North Africa that depict men fishing with nets. Pliny, and other Roman scholars, describe garum (fish sauce) production, and its remains have been found in amphorae from Pompeii (Carannante 2019). The fish used to make Pompeii garum were common to the shallow waters of the Mediterranean (Carannante 2019). Unlike the remains of terrestrial animals, fish bones are not found at most Roman sites, and some have argued fish consumption was limited to people of higher status (e.g., Locker 2007).
Floral and faunal remains recovered from Herculaneum and nearby villages point to an array of available foods, but a relatively small number were widely exploited. Being a part of the empire meant the Herculaneum people had access to foods imported from Europe, Asia, and Africa in addition to those produced locally. Evidence for this broad-based subsistence is best preserved at sites that were buried by the eruption of Vesuvius including Herculaneum, Pompeii, and Boscoreale. Even perishable items, including complete loaves of bread, have been found at these sites. Foods carbonized or mineralized by the eruption include wheat, barley, and foxtail millet, as well as walnuts, grapes, figs, dates, prunes, field pansies, vetch, squash, cantaloupe, asparagus, lentils, pine, black pepper, and olives (e.g., Borgongino 2006; Rowan 2014).
Wine was an important resource throughout the empire (Figure 3), and numerous amphorae of wine were found at Herculaneum. Faunal remains preserved by the eruption include imported animals such as ostrich, as well as more locally available animals including boar, deer, chicken, geese, and bears (Waldstein and Shoobridge 1908; Robinson and Rowan 2015). Eggs from chickens and geese were preserved, too (Rowan 2014). Basalt grinding mills for the processing of cereal grains are common at Herculaneum. Additional evidence for food processing includes the presence of large cooking ovens, stored salt, and butchering tools (Waldstein and Shoobridge 1908; Robinson and Rowan 2015).

Marine resource exploitation is clear at Herculaneum. Rowan (2014, 2017) and Nicholson et al. (2018) found evidence for the consumption of dozens of invertebrate and vertebrate species including limpet, clams, scallops, sea breams, damselfish, horse mackerel, and anchovies in sewers linked to housing for low- and moderate-income people. They identified 45 fish and 53 shellfish species in the sewer deposits (Rowan, Flohr, and Wilson 2017, p 277). Rowan, Flohr, and Wilson (2017) also found evidence of cereal grain and fruit exploitation and determined the people of Herculaneum had enough food to meet their nutritional demands regardless of their socioeconomic position.
Diet is that part of a population's subsistence that contributes to their nutritional needs, that is, it is the food that is consumed (Hillson 1979). Thus, evidence for ancient diets comes via the study of human remains and coprolites. Because Rowan's work on the Herculaneum sewers includes human feces, that is, coprolites, it reflects diet. However, sewers contain materials that did not necessarily pass through a human digestive tract, for example food production waste thrown away by shops or bakeries. For this reason, the dietary reconstructions discussed here focus on stable isotope values derived from human skeletons (e.g., Prowse et al. 2004, 2005; Martyn et al. 2018).
Herculaneum is a coastal city, however, its isotopic data—drawn from the non-Bisel/Capasso Herculaneum skeletons—indicate that terrestrial resources were more important dietarily than marine resources; they made up at least 70% of the diet, (Martyn et al. 2018). The coastal site of Velia in southern Italy, which was a contemporary of Herculaneum, has strong isotopic evidence for a terrestrial-based diet rich in cereal grains, but little evidence for meat consumption (Craig et al. 2009). Similarly, isotopic data from the inland sites of Michelet Necropolis in France and the ANAS site in Rome indicate terrestrially-based diets. For the latter, the isotopic signatures point to the consumption of cereals, legumes, fruits, and vegetables (Prowse et al. 2004; Avery et al. 2021, 2023).
Roman fish consumption was present throughout the empire, but rarely was it a dominant part of the diet. The people of the site of Poundbury in England, which is near, but not on the coast, relied heavily on fish, based on their relatively high stable isotope values (Richards et al. 1998). The people of the coastal city of Isola Sacra near Rome, also had relatively high stable nitrogen (δ15N) and carbon (δ13C) values indicative of marine fish consumption (Prowse et al. 2004). Yet, even at Isola Sacra, which has some of the strongest evidence for fish consumption, the apatite δ13C values indicate a total dietary carbon intake that was primarily from terrestrial foods. The same is true for the people of the late Roman coastal site of Leptiminus, Tunisia whose isotopes indicate they relied primarily on terrestrial resources despite consuming sizable quantities of fish (Keenleyside et al. 2009). In fact, regardless if a Roman city is coastal or inland, the isotopic data indicate terrestrial resources were more important dietarily than marine resources. Among the terrestrial foods, meat consumption is commonly, but not always indicated (e.g., Craig et al. 2009). Isotopic evidence for the consumption of terrestrial plants, such as cereal grains, is ubiquitous.
2.1 Diet in the Context of the Life Course
Access to and the consumption of foods may change as humans transition from early childhood through late adulthood. Likewise, sex (and gender) impact what people eat and the life course, therefore, serves as a scaffold upon which dietary reconstructions are contextualized (e.g., Prowse 2011). Reasons for age and sex-based dietary variations include, for example, musculoskeletal differences between young subadults and older subadults/adults, health, personal preferences, social norms, and social status (e.g., Elder Jr. 1998; Goodman and Leatherman 1998; Prowse et al. 2004, 2005; Sobal et al. 2006; Beauchesne and Agarwal 2018; Kelly, Schmidt, and D'Anastasio 2020; Avery et al. 2023). The focus of the current paper is intra-populational variations in diet, with age and sex serving as skeletally discernible life course attributes. Social attributes, including social status, are not explicitly indicated among the people found at Herculaneum, but they are potential factors that affected food consumption.
Roman dietary reconstructions based on stable isotopes draw from life course theory (e.g., Prowse 2011), and it is clear Roman diets differed by age and sex, although not uniformly. At the Late Roman Michelet Necropolis in France, Avery et al. (2021) found that from about age 5 to the late teens, meat or animal byproducts (like cheese) became increasingly important. They also found that male and female subadults had similar isotopic values up until about age 16, when female δ15N values started to exceed those of males. Avery et al. (2021) attribute this to gender roles that led males into careers away from home where they encountered different foods. Females, on the other hand, tended to remain at home where they consumed foods that were isotopically similar to what they ate as subadults. At Leptiminus in Tunisia, there were no differences based on sex, but isotope values differed between adults and subadults, the latter having a greater dependence on terrestrial plants (Keenleyside et al. 2009). At Velia in southern Italy, males ate more higher trophic level fish than did the females (Craig et al. 2009).
Prowse et al. (2004, 2005) found age-based dietary differences at Isola Sacra. The collagen of older individuals was significantly enriched in 15N but not 13C. Bone carbonate was somewhat depleted in 13C in some older individuals, suggesting increased consumption of olive oil and possibly wine. Overall, the ranges of carbon and nitrogen values increase with older people, indicating that dietary breadth increased with age (Prowse et al. 2005). Subadults under 5 years old appear to have consumed primarily terrestrial foods. In fact, most subadults had low meat diets dominated by terrestrial plants, presumably cereal grains. The adults had the inverse, indicating more meat and fish consumption. Interestingly, those who consumed larger quantities of marine resources also consumed more wine and olive oil, perhaps underscoring a relationship between age and status. Isola Sacra males consumed more marine resources than the females, although both males and females consumed fish, and older females consumed more fish than younger females. The sex-based differences could relate to status differences for males and females in androcentric Roman society (Prowse et al. 2005). At Herculaneum, males have isotopic evidence of consuming more high trophic level marine resources compared to females. Age was less of a factor, although those over 30 had significantly higher δ15N values, due primarily to the males (Martyn et al. 2018).
While the examples provided above are not exhaustive, they are sufficient to indicate strong evidence for age and sex affecting Roman dietary variation. An aspect of life course focused dietary studies is location, that is, where people live. For example, living near a coast or further inland can affect access to foods. Thus, some of the isotopic studies discussed here compared people from different locations (e.g., Martyn et al. 2018). Unfortunately, dental microwear texture data are currently unknown from other Roman sites, although efforts are underway to rectify this; the emphasis here, therefore, is solely on the people of Herculaneum.
2.2 Dental Microwear Texture Analysis (DMTA)
Like isotopic analysis, DMTA is a means of reconstructing ancient diets; although initially developed for non-human primates and hominins, it has been applied to humans from archaeological contexts as well (e.g., Bas et al. 2020, 2023; Droke et al. 2020; Da Gloria and Schmidt 2020; El Zaatari 2008, 2010; El Zaatari et al. 2016; El Zaatari and Hublin 2014; Holmes, Moore and Schmidt 2024; Kelly, Schmidt, and D'Anastasio 2020; Mahoney et al. 2016; Mahoney et al. 2019; Schmidt 2018, 2021; Schmidt et al. 2016; Schmidt et al. 2019; Schmidt et al. 2020; Williams, Schmidt, and Droke 2020). It borrows from engineering surface metrology and characterizes microscopic surface contours (Schmidt and Ungar 2023). For example, surfaces may have few changes in relief, or they may be markedly uneven; surface features, like scratches and pits, may be ordered or haphazard. Standardized computations allow surface metrologists to precisely quantify surface characteristics. Surface characters, however, can differ based on the scale of observation; consider the blade edge of a steel knife that looks smooth through a hand lens, but which has numerous peaks and valleys under high magnification.
To address differences in surface textures (i.e., the aggregate of surface peaks and valleys) at different scales, dental microwear texture analysts use fractal-based computations (see Scott et al. 2006; Scott et al. 2005). These calculations include measurements taken at multiple scales and/or allow analysts to specify the scales within which they work. The programs initially employed for DMTA were Toothfrax and Sfrax; these were based on scale-sensitive fractal analysis and analysts used standardized settings for data collection. These programs are no longer available as free-standing entities, and both have been incorporated into MountainsMap (as well as SolarMap) software.
DMTA in humans provides dietary signatures, termed “foodprints,” created by foods contacting dental chewing surfaces during mastication (Ungar 2011; Schmidt and Ungar 2023). Foodprints are determined, at least in part, by the food's mechanical properties, which are the ways by which materials behave when placed under a load (Berthaume 2016, p 80). Brittle foods, like seeds and nuts are thought to generate pitting and coarser surface topographies (e.g., Van Casteren et al. 2020). Tough foods, like fibrous plants, may require several chewing cycles to break down (e.g., Scott et al. 2012). Traditionally, microwear texture analysts use the terms “hard” and “tough” nearly synonymously for brittle and ductile, although they are not true synonyms (Lucas 2004; Berthaume 2016). Following dental microwear convention, the term “hard,” is used herein to indicate a diet that creates high complexity values. The term “tough” refers to diets that generate high anisotropy. Informally, the term “soft foods” is used as a contrast to “hard foods” although soft is not a mechanical property (Berthaume 2016). Another informal term used to characterize microwear is “abrasive,” which relates to microwear associated with marked gross occlusal wear (e.g., Schmidt 2010), or microwear having a high number of sizable microwear features (e.g., Scott et al. 2012; Teaford et al. 2021). Often abrasive diets are attributed to contamination of foods by exogenous grit, although more compliant foods mitigate the affect grit has on the enamel (e.g., Hua, Chen, and Ungar 2020). In humans, dietary abrasiveness often reflects the degree to which food is processed. Poorly processed wild foods tend to lead to extreme macrowear and numerous microwear striae. Non-abrasive diets tend to be found among farming groups who used stone or wooden grinding tools and in pastoral groups who fed primarily on meat products (e.g., Schmidt 2010; Schmidt et al. 2016).
However, the creation of microwear features is complex and not simply an artifact of food properties or food preparation (e.g., van Casteren et al. 2018). Foodprints also depend on the properties of dental enamel. Enamel is a composite material; it contains crystallites of hydroxyapatite bound by non-collagenous proteins (Lei et al. 2020). Because of this, foods do not have to be as hard as enamel to create dental microwear features; what is needed is food particles with angled, or polyhedral, surfaces capable of dislodging crystallites (Xia et al. 2015). Thus, an array of foods can generate similar microwear features (e.g., Hua et al. 2015) and foodprints, as they are currently understood, are not indicators of particular foods; it is necessary to draw inferences from subsistence records to interpret human dental microwear textures (El Zaatari 2010; Ungar et al. 2012; Schmidt et al. 2019).
There are several standard calculations used to determine surface texture; in DMTA, the most common include complexity, anisotropy, scale of maximum complexity, and textural fill volume (e.g., Scott et al. 2005, 2006). Complexity indicates surface coarseness. A smooth surface has a lower complexity while a surface with more peaks and valleys has a higher complexity. Anisotropy indicates feature orientation similarity. A surface with high anisotropy has its features in a common orientation; surfaces with low anisotropy have features going in multiple directions. Scale of maximum complexity (Smc) is related to complexity. Recall that fractal computations consider surface textures at multiple scales. The Smc value is the scale where the complexity value is greatest. Textural fill volume (Tfv) is a measurement of the voids in the surface. Higher Tfv values indicate more of the surface is missing (see Scott et al. 2005, 2006 for details regarding the specific calculations).
People consuming hard seeds or nuts, tend to have higher complexity values and lower anisotropy, whereas those eating softer or tougher foods, such as highly processed cereals or cooked meat, tend to have lower complexity and higher anisotropy (e.g., Karriger, Schmidt and Smith 2016; Schmidt et al. 2016; Schmidt et al. 2019). Human dental microwear values tend to vary within groups, although patterns emerge. For example, El Zaatari (2010) found that foragers thought to consume high amounts of fish had low complexity and high anisotropy values. In contrast, she also found that arctic foragers had elevated complexity values (El Zaatari 2010). Elevated complexity values are not limited to foragers. Late pre-Colonial farmers of Indiana had high complexity values thought to be caused by the consumption of wild nuts in addition to their maize-based diet (Holmes, Moore and Schmidt 2024).
In humans, the degree of food processing affects complexity (Schmidt et al. 2019). Lightly processed foods, like modestly or unground seeds or nuts, small bones, small invertebrate shell fragments, and grit, tend to elevate complexity values (see El Zaatari 2010; Ungar 2011; Ungar et al. 2012; Teaford et al. 2021). Those that are highly processed, like those of maize or wheat farmers, tend to have lower complexity (Schmidt et al. 2019). An exception to this pattern is meat (i.e., vertebrate muscle tissue), which generates no microwear when consumed in isolation (e.g., Hua et al. 2015). Meat is a tough food, meaning it is not brittle or susceptible to rapid breakdown in the mouth (see Hua et al. 2015). Its consumption in humans, therefore, tends to lead to low complexity values unless it is consumed along with a sizable amount of contaminants (e.g., El Zaatari 2010; Hua, Chen, and Ungar 2020).
In humans, higher anisotropy values are thought to be associated with dietary homogeneity (Schmidt et al. 2019). Diets made up of foods having similar chewing requirements, lead to similarly oriented mandibular movements, which leads to the formation of microwear striae coursing in the same direction. Consider the meat example above; humans who eat high levels of meat can have high anisotropy values (e.g., El Zaatari 2010). Examples of foods leading to high anisotropy values go behind meat; diets consisting of foods that are tough tend produce repetitive chewing motions and similarly oriented features. Diets comprised of hard foods tend to require the mandible to move in multiple directions to breakdown the ever-reducing hard food bolus. This leads to a decrease in anisotropy as microwear features form in multiple directions (see Schmidt et al. 2019).
Textural fill volume is quite varied in humans; higher Tfv values are often associated with higher complexity values (El Zaatari 2010), but not always (e.g., Schmidt 2021). In fact, it is rare for microwear texture variables to correlate. Generally, Tfv indicates a diet that is coarser and generates larger features. Likewise, it is thought that scale of maximum complexity tends to be associated with particle size (Ungar et al. 2012). In humans, more processed diets are likely to have smaller features and, therefore, greater complexity at finer scales.
Finally, dental microwear is a continuous process, whereby newer microwear features replace older ones (e.g., Teaford and Oyen 1989; Teaford 1994). Therefore, it tends to represent a relatively brief portion of a person's life, usually counted in weeks or months, unlike macrowear, which is gross occlusal wear formed over a lifetime of a person's dental hard tissue loss (e.g., Watson and Schmidt 2019). This makes microwear sensitive to seasonal changes in diet, dietary changes caused by chronic disease states just prior to death (e.g., Casserly et al. 2014) and, in the case of Herculaneum, an indicator of intra-populational dietary breadth at a single point in time.
The objective of the current study is to characterize dietary patterns among the people of Herculaneum by age and sex via DMTA. The Roman subsistence and dietary records point to diverse diets for Roman people that included terrestrial and marine foods, with terrestrial plants and animals tending to be favored (e.g., Martyn et al. 2018). Moreover, the isotopic data indicate dietary differences based on age and sex. The specific hypotheses tested herein are described below, but, in short, there is an expectation that Herculaneum subadult diets changed as they got older, that subadults and adults had different diets, and that adult males and females had different diets.
3 Materials
The current study is of dental casts from 58 adults (age 16+) and 23 subadults (age 0–16) from Herculaneum. All are housed at the University Museum, Chieti, Italy. The 81 people included here are those for whom we successfully collected DMTA data (the museum houses 153 individuals). Sex estimations for the adults produced 27 females and 31 males. Here we define subadults based on the five age stages of Roman life (Harlow and Laurence 2002), who note Roman childhood was a brief period and teens entered adult social roles by the age of 16 when men would go into military service, and women would take on more domestic roles. Young people sometimes married as early as 12 or 13, but typically marriage and other adult activities took place a few years later. For the current study, therefore, subadults range in age from 0 to 15.
All people studied here died during the eruption of Vesuvius in A.D. 79. The relatively modest thermal damage to the teeth meant that most people retained excellent dental microwear. Individuals for whom we were unable to get microwear data included those who had unerupted teeth, those having their occlusal surfaces covered with charred soft tissues, and those having molars that were too friable for molding.
3.1 Methods
The DMTA followed standard procedures (e.g., Scott et al. 2005; Ungar et al. 2012). We collected DMTA data from one molar from each person, an upper or lower first or second molar. Analysts have found that first and second molars from the maxilla and mandible tend to produce comparable microwear, likely due to their similar roles in mastication (e.g., Teaford 1994; Ungar 2015). Third molars are suitable for microwear but can have elevated complexity values due to their distal positions in the mouth where chewing forces can be high and transverse mandibular movement somewhat limited (Perash 2018; Edmonds and Glowacka 2020).
The study includes deciduous premolars (aka molars). Adult enamel is thicker and bears a greater percentage of calcium and phosphorous compared to deciduous enamel (De Menezes Oliveira et al. 2010; Mahoney 2010) and overall adult enamel is considered more mineralized (Wilson and Beynon 1989). Nonetheless, deciduous enamel is suitable for microwear provided analysts consider factors in addition to diet that can affect the presence of microwear features (e.g., Mahoney et al. 2016; Scott and Halcrow 2017; Estalrrich and Krueger 2022; Bas et al. 2020, 2023). An important factor is chewing mechanics in young subadults. People under the age of 5 lack both the bite force and movement found in older people. This gives the very young a more vertical movement of the mandible and the potential for elevated complexity values (e.g., Scott and Halcrow 2017; Mahoney et al. 2016). Kelly, Schmidt, and D'Anastasio (2020) conducted a study of the deciduous and adult teeth from the Herculaneum subadults to determine if there were significant differences between them. They reported no difference, which led to the inclusion of deciduous teeth in the current study.
Dental replication used President Jet polyvinylsiloxane for molds and Four to One Super Hard Epoxy resin for the casts. Dental surface data collection employed a Sensofar Plu 2300 white-light confocal profiler with a X-Y resolution of 0.17 μm and a vertical resolution of 0.20 μm at 100X magnification. Data collection focused on Phase II occlusal facets. Preparation of the data clouds for analysis began by leveling using a least squares algorithm. Surface contamination was removed digitally, and data voids were filled. Analysis used Toothfrax and Sfrax software (acquired prior to their discontinuation) to calculate surface textures via complexity, anisotropy, Tfv, and Smc.
Age and sex estimates are from Capasso (2001) and confirmed visually at the time of tooth molding. Point ages are an average of each age range estimate in Capasso (2001). Subadult age groups are 0–5, 6–10, and 11–15. For the adults, grouping by age follows Standards (1994), however, unlike Standards (1994) we include 16 to18-year-olds in the youngest adult age group, giving it an age range of 16–34. The other adult age groups are 35–50, and 50+. These age groups are largely arbitrary constructs and are employed herein to explore nuances of life stages (i.e., early, middle, late childhood and early, middle, late adulthood). For the current study, the term subadult is used to describe all individuals aged 15 and under.
3.2 Statistical Procedures
In general, p-values in frequentist statistics do not provide strengths of relationships among variables within a statistical model. Improvements to null-hypothesis statistical testing (NHST) include the use of effect indicators and their 95% confidence intervals to indicate significance (e.g., Smith 2018). Scholars, however, are increasingly abandoning NHST constructs entirely and are migrating to Bayesian statistics (McElreath 2018). Predicated on the idea that prior conditions can affect outcomes, this approach considers the relative predictive viability of two or more hypotheses and provides a calculation of a particular hypothesis' relative plausibility called the Bayes factor (BF) (Van Doorn et al. 2021).
The Bayesian statistical process begins with relative prior odds, generates a Bayes factor from the data, and finally indicates the posterior odds of the competing hypotheses (Figure 4). In the end, it provides relative odds as well as the explanatory robustness of each hypothesis. For example, when comparing an alternative hypothesis to a null hypothesis, a BF of 5 indicates the alternative hypothesis' relative plausibility is 5 times greater than that of the null hypothesis. In the current study, all Bayesian results are set to have the alternative hypothesis compared to the null hypothesis, therefore, all Bayes Factors for the null condition will be 1.00 and the BF for the alternative will provide the plausibility of the alternative hypothesis.

Interpreting BF magnitude depends on the study, but generally, a BF between 1 and 3 is considered weak or anecdotal. Bayes Factors between 3 and 10 are considered moderate, and those over 10 are considered strong. A BF of 1 means the alternative and null hypotheses are equally plausible. Bayes factors between 1 and 1/3 give anecdotal evidence for the null hypothesis, 1/3–1/10 give moderate evidence and 1/10 to 1/30 provide strong evidence for the null hypothesis. For the current study, outcomes that produce a BF in favor of the alternative hypothesis that exceed 1.5 are explored and those tests having a BF above 3 are considered meaningfully significant.
The statistical tests employed here include Bayesian ANOVA, regression, and correlation with uniform prior probabilities (e.g., Faulkenberry, Ly, and Wagenmakers 2020; van den Bergh et al. 2020). Additionally, the study applies classification procedures including binary linear regression (BLR), for sorting of two groups, and discriminant function analysis (DFA) with leave-one-out cross-validation, for sorting more than two groups. We used JASP 0.18.1 (JASP Team 2023) for the Bayesian computations and SPSS 28 for the BLR and DFA. The complexity, anisotropy, and Tfv data were normally distributed but Smc was not, which required a rank-transformation. Ranked Smc data are abbreviated RSmc.
3.3 Hypotheses
There are three working hypotheses, each with specific alternative hypotheses (HA) we tested against the null condition.
Hypothesis 1.Dental microwear textures varied among the subadults. Rationale: Subsistence and dietary records point to very young subadults consuming cereal grain-based porridges and older subadults taking on a more adult diet. If the Herculaneum youngest subadults had soft, homogenous diets, then: HA1: Subadult complexity should increase with age; HA2: Subadult anisotropy should decrease with age; HA3: Subadult RSmc should increase with age; HA4: Subadult Tfv should increase with age.
Hypothesis 2.Subadult dental microwear textures differed from that of the adults. Rationale: Isotopic data point to subadults having a more homogenous, less processed diet based on plants and the adults eating a wider array of foods including more meat and fish. If the Herculaneum subadults consumed these foods, then: HA5: Subadult complexity should be higher than that of the adults; HA6: Subadult anisotropy should be higher than that of the adults; HA7: Subadult RSmc should be higher than that of the adults; HA8: Subadult Tfv should be lower than that of the adults.
Hypothesis 3.Adult dental microwear textures differed by age and sex. Rationale: Isotopic data point to changes in diet by sex with age. If the Herculaneum adult diets changed as they aged, then: HA9: Male and female complexity should decrease with age; HA10: Male and female anisotropy should decrease with age; HA11: Male and female RSmc should decrease with age; The null condition for Tfv should remain the same regardless of age (the increase in meat and/or fish consumption should not affect periodic impacts of harder and/or abrasive foods).
4 Results
4.1 Hypothesis 1 (HA1–4). Dental Microwear Textures Varied Among the Subadults
For the subadults, the summary data indicate that complexity values are static across the three subadult age groups (e.g., 0–5, 6–10, 11–15), with each group averaging around 1.5 (Table 1).
| Subadult age groups | Complexity | Anisotropy | RSmc | Tfv | |
|---|---|---|---|---|---|
| 0–5 | Mean | 1.59 | 0.0059 | 17.0 | 38525.9 |
| N | 5 | 5 | 5 | 5 | |
| SD | 0.37 | 0.0032 | 6.7 | 2297.6 | |
| 6–10 | Mean | 1.52 | 0.0037 | 9.40 | 35182.7 |
| N | 10 | 10 | 10 | 10 | |
| SD | 0.74 | 0.0023 | 6.17 | 12069.7 | |
| 11–15 | Mean | 1.58 | 0.0045 | 14.88 | 34161.6 |
| N | 8 | 8 | 8 | 8 | |
| SD | 0.48 | 0.0019 | 6.27 | 10071.8 | |
Bayesian ANOVA found no differences based on age group for any of the DMTA values (Table 2). For linear relationships, Bayesian regressions addressed point age estimate and each DMTA variable. Again, there are no relationships (Table 3). Similarly, a discriminant function analysis failed to meaningfully sort subadults by age; successful classifications by age group were: 0–5 had 40%; 6–10 had 60%, and 11–15 had 50% (Table 4). Overall, the assertions of Hypothesis 1 are not supported.
| Complexity | P (M) | P (M|data) | BFM | BF10 | Error % |
|---|---|---|---|---|---|
| Null model | 0.50 | 0.79 | 3.84 | 1.00 | |
| Subadult age groups | 0.50 | 0.21 | 0.26 | 0.26 | 0.04 |
| Anisotropy | |||||
| Null model | 0.50 | 0.64 | 1.77 | 1.00 | |
| Subadult age groups | 0.50 | 0.36 | 0.56 | 0.56 | 0.02 |
| Smc (ranked) | |||||
| Null model | 0.50 | 0.41 | 0.70 | 1.00 | |
| Subadult age groups | 0.50 | 0.59 | 1.42 | 1.42 | 0.01 |
| Tfv | |||||
| Null model | 0.50 | 0.77 | 3.32 | 1.00 | |
| Subadult age groups | 0.50 | 0.23 | 0.30 | 0.30 | 0.03 |
| Models | P (M) | P (M|data) | BFM | BF10 | R 2 |
|---|---|---|---|---|---|
| Null model | 0.20 | 0.47 | 3.53 | 1.00 | 0.00 |
| Complexity + Anisotropy + Tfv + Smc | 0.20 | 0.07 | 0.32 | 0.16 | 0.12 |
| Smc | 0.05 | 0.07 | 1.41 | 0.59 | 0.06 |
| Anisotropy | 0.05 | 0.05 | 1.10 | 0.47 | 0.03 |
| Complexity | 0.05 | 0.05 | 0.99 | 0.42 | 0.01 |
| Tfv | 0.05 | 0.05 | 0.94 | 0.40 | 0.01 |
| Anisotropy + Smc | 0.03 | 0.03 | 1.02 | 0.43 | 0.11 |
| Complexity + Anisotropy + Smc | 0.05 | 0.03 | 0.58 | 0.25 | 0.12 |
| Anisotropy + Tfv + Smc | 0.05 | 0.03 | 0.56 | 0.24 | 0.11 |
| Complexity + Smc | 0.03 | 0.02 | 0.70 | 0.30 | 0.06 |
| Tfv + Smc | 0.03 | 0.02 | 0.69 | 0.30 | 0.06 |
| Complexity + Anisotropy | 0.03 | 0.02 | 0.63 | 0.27 | 0.05 |
| Complexity + Tfv + Smc | 0.05 | 0.02 | 0.40 | 0.18 | 0.07 |
| Complexity + Anisotropy + Tfv | 0.05 | 0.02 | 0.38 | 0.17 | 0.06 |
| Anisotropy + Tfv | 0.03 | 0.02 | 0.58 | 0.25 | 0.04 |
| Complexity + Tfv | 0.03 | 0.02 | 0.53 | 0.23 | 0.02 |
| Subadult age groups | Predicted Group Membership | Total | ||||
|---|---|---|---|---|---|---|
| 0–5 | 6–10 | 11–15 | ||||
| Original | Count | 0–5 | 2 | 2 | 1 | 5 |
| 6–10 | 2 | 7 | 1 | 10 | ||
| 11–15 | 0 | 2 | 6 | 8 | ||
| % | 0–5 | 40.0 | 40.0 | 20.0 | 100.0 | |
| 6–10 | 20.0 | 70.0 | 10.0 | 100.0 | ||
| 11–15 | 0.0 | 25.0 | 75.0 | 100.0 | ||
| Cross-validatedb | Count | 0–5 | 2 | 2 | 1 | 5 |
| 6–10 | 2 | 6 | 2 | 10 | ||
| 11–15 | 1 | 3 | 4 | 8 | ||
| % | 0–5 | 40.0 | 40.0 | 20.0 | 100.0 | |
| 6–10 | 20.0 | 60.0 | 20.0 | 100.0 | ||
| 11–15 | 12.5 | 37.5 | 50.0 | 100.0 | ||
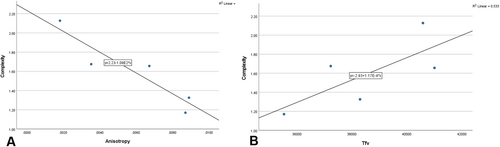
While linear relationships failed across the three subadult age groups, two DMTA relationships emerged within the youngest age group (Figure 5). Complexity has an inverse correlation with anisotropy (r2 = −0.924, BF10 = 3.61) and a positive relationship with Tfv (r2 = 0.853; BF10 = 1.22 [stretched beta prior widths = 1]). The BF10 for the complexity—Tfv correlation is weak, which surely is affected by the small sample size of five.
4.2 Hypothesis 2 (HA5–8). Subadult Dental Microwear Textures Differed From That of the Adults
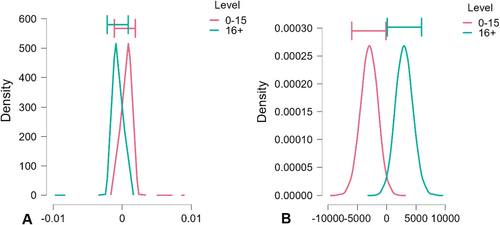
The summary data indicate the mean for adult complexity is very similar to that of the subadults, but the difference in anisotropy between adults and subadults is vivid (Table 5). Bayesian ANOVA found no difference in complexity, but there is a significant BF10 of 7.59 for anisotropy. There was no significant difference in RSmc; Tfv had a modestly significant BF10 of 2.23 (see Table 6 and Figure 6). A binary logistic regression including all four DMTA variables successfully classified 43.5% of the subadults and 96.5% of the adults (Table 7).
| Complexity | Anisotropy | RSmc | Tfv | ||
|---|---|---|---|---|---|
| Sub-adults | Mean | 1.55 | 0.0045 | 43.5 | 35554.3 |
| N | 23 | 23 | 23 | 23 | |
| SD | 0.57 | 0.0024 | 25.7 | 9777.9 | |
| Adults | Mean | 1.47 | 0.0031 | 40.0 | 42368.4 |
| N | 58 | 58 | 58 | 57 | |
| SD | 0.59 | 0.0017 | 22.8 | 12882.5 | |
| Models | P (M) | P (M|data) | BFM | BF10 | Error % |
|---|---|---|---|---|---|
| Complexity | |||||
| Null model | 0.50 | 0.78 | 3.45 | 1.00 | |
| Subadult—adult | 0.50 | 0.22 | 0.29 | 0.29 | 0.01 |
| Anisotropy | |||||
| Null model | 0.50 | 0.12 | 0.13 | 1.00 | |
| Subadult—adult | 0.50 | 0.88 | 7.59 | 7.59 | 5.08 × 10−7 |
| RSmc (rank transformed) | |||||
| Null model | 0.50 | 0.77 | 3.41 | 1.00 | |
| Subadult—adult | 0.50 | 0.23 | 0.29 | 0.29 | 0.01 |
| Tfv | |||||
| Null model | 0.50 | 0.31 | 0.45 | 1.00 | |
| Subadult—adult | 0.50 | 0.69 | 2.23 | 2.23 | 8.14 × 10−3 |
| Observed | Predicted | |||
|---|---|---|---|---|
| Subadults and adults | Percentage correct | |||
| 0–15 | 16+ | |||
| Subadults adults | 0–15 | 10 | 13 | 43.5 |
| 16+ | 2 | 55 | 96.5 | |
| Overall percentage | — | — | — | 81.3 |
4.3 Hypothesis 3 (HA9–11). Adult Dental Microwear Textures Differed by Age and Sex
The summary data indicate the mean male complexity is larger than that of the females, 1.59–1.34, respectively. Anisotropy, RSmc, and Tfv means are very similar. Male ranges are greater for complexity, anisotropy, and RSmc; they are similar for Tfv (Table 8) and there are no differences between the sexes when age is not controlled (Table 9).
| Complexity | Anisotropy | RSmc | Tfv | ||
|---|---|---|---|---|---|
| Male | Mean | 1.59 | 0.0032 | 27.9 | 41421.7 |
| N | 31 | 31 | 31 | 31 | |
| SD | 0.66 | 0.0018 | 16.9 | 13076.0 | |
| Female | Mean | 1.34 | 0.0031 | 31.3 | 43497.2 |
| N | 27 | 27 | 27 | 26 | |
| SD | 0.48 | 0.0017 | 17.1 | 12811.4 | |
| Models | P (M) | P (M|data) | BFM | BF10 | Error % |
|---|---|---|---|---|---|
| Complexity | |||||
| Null model | 0.50 | 0.57 | 1.32 | 1.00 | |
| Sex | 0.50 | 0.43 | 0.76 | 0.76 | 9.14 × 10−3 |
| Anisotropy | |||||
| Null Model | 0.50 | 0.79 | 3.73 | 1.00 | |
| Sex | 0.50 | 0.21 | 0.27 | 0.27 | 9.61 × 10−3 |
| Smc | |||||
| Null model | 0.50 | 0.79 | 3.73 | 1.00 | |
| Sex2 | 0.50 | 0.21 | 0.27 | 0.27 | 9.61 × 10−3 |
| Tfv | |||||
| Null model | 0.50 | 0.76 | 3.20 | 1.00 | |
| Sex2 | 0.50 | 0.24 | 0.31 | 0.31 | 9.18 × 10−3 |
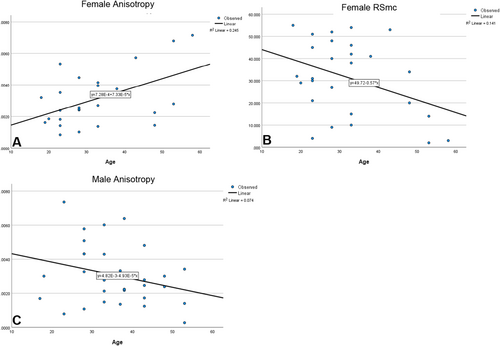
Among females, point age and anisotropy significantly positively covary (BF10 5.72); older females have greater anisotropy than younger females. Likewise, in males there is a linear relationship between age and anisotropy, although it is an inverse relationship. Older males have less similarity in feature orientation compared to younger males and the male relationship between age and anisotropy is weaker than that of the females (BF10 = 2.21). Females have a weak relationship between point age and RSmc (Tables 10 and 11; Figures 7-9).
| Complexity | P (M) | P (M|data) | BFM | BF10 | R 2 |
|---|---|---|---|---|---|
| Null model | 0.50 | 0.57 | 1.33 | 1.00 | 0.00 |
| Point age | 0.50 | 0.43 | 0.75 | 0.75 | 0.07 |
| Anisotropy | |||||
| Null model | 0.50 | 0.15 | 0.17 | 1.00 | 0.00 |
| Point age | 0.50 | 0.85 | 5.72 | 5.72 | 0.25 |
| RSmc | |||||
| Null model | 0.50 | 0.39 | 0.65 | 1.00 | 0.00 |
| Point age | 0.50 | 0.61 | 1.55 | 1.55 | 0.14 |
| Tfv | |||||
| Null model | 0.50 | 0.70 | 2.35 | 1.00 | 0.00 |
| Point age | 0.50 | 0.30 | 0.43 | 0.43 | 0.02 |
| Complexity | P (M) | P (M|data) | BFM | BF10 | R 2 |
|---|---|---|---|---|---|
| Null model | 0.50 | 0.74 | 2.87 | 1.00 | 0.00 |
| Point age | 0.50 | 0.26 | 0.35 | 0.35 | 0.00 |
| Anisotropy | |||||
| Null model | 0.50 | 0.31 | 0.45 | 1.00 | 0.00 |
| Point age | 0.50 | 0.69 | 2.21 | 2.21 | 0.15 |
| RSMC | |||||
| Null model | 0.50 | 0.73 | 2.69 | 1.00 | 0.00 |
| Point age | 0.50 | 0.27 | 0.37 | 0.37 | 0.01 |
| Tfv | |||||
| Null model | 0.50 | 0.54 | 1.19 | 1.00 | 0.00 |
| Point age | 0.50 | 0.46 | 0.84 | 0.84 | 0.08 |
To improve our understanding of the role of age in DMTA expression, we conducted discriminant function analysis for each age group by sex. This allowed us to see if age groups within each sex had diets unique to them. The best classification models for both males and females included all four DMTA variables and the output discussed here come from the leave one out cross-validated DFA classifications. For females, classification sorted the young adults (age 16–34) rather well; about 83% were properly classified (15 of 18). For the middle age group (35–50), about 40% (2 of 5) were classified properly. Of the three old adults (those over 50), 2 of the 3 were properly classified. The overall classification success rate was 73.1% (Table 12). In contrast, only 45.2% of the males could be successfully classified by age group. About 67% of the young adult males (10 of 15) and 30% of the middle adult males (4 of 13) were properly classified. None of the three old adult males were classified properly (Table 13).
| Adult age group | Predicted group membership | Total | ||||
|---|---|---|---|---|---|---|
| 16–35 | 36–50 | 51+ | ||||
| Original | Count | 16–34 | 15 | 2 | 1 | 18 |
| 35–49 | 3 | 2 | 0 | 5 | ||
| 50+ | 1 | 0 | 2 | 3 | ||
| % | 16–34 | 83.3 | 11.1 | 5.6 | 100.0 | |
| 35–49 | 60.0 | 40.0 | 0.0 | 100.0 | ||
| 50+ | 33.3 | 0.0 | 66.7 | 100.0 | ||
| Cross-validatedb | Count | 16–34 | 15 | 2 | 1 | 18 |
| 35–49 | 3 | 2 | 0 | 5 | ||
| 50+ | 1 | 0 | 2 | 3 | ||
| % | 16–34 | 83.3 | 11.1 | 5.6 | 100.0 | |
| 35–49 | 60.0 | 40.0 | 0.0 | 100.0 | ||
| 50+ | 33.3 | 0.0 | 66.7 | 100.0 | ||
| Adult age group | Predicted group membership | Total | ||||
|---|---|---|---|---|---|---|
| 16–34 | 35–49 | 50+ | ||||
| Original | Count | 16–34 | 10 | 5 | 0 | 15 |
| 35–49 | 6 | 7 | 0 | 13 | ||
| 50+ | 0 | 3 | 0 | 3 | ||
| % | 16–34 | 66.7 | 33.3 | 0.0 | 100.0 | |
| 35–49 | 46.2 | 53.8 | 0.0 | 100.0 | ||
| 50+ | 0.0 | 100.0 | 0.0 | 100.0 | ||
| Cross-validatedb | Count | 16–34 | 10 | 4 | 1 | 15 |
| 35–49 | 9 | 4 | 0 | 13 | ||
| 50+ | 0 | 3 | 0 | 3 | ||
| % | 16–34 | 66.7 | 26.7 | 6.7 | 100.0 | |
| 35–49 | 69.2 | 30.8 | 0.0 | 100.0 | ||
| 50+ | 0.0 | 100.0 | 0.0 | 100.0 | ||
Bayesian ANOVAs with adult age group and sex as the independent variables, determined a significant interaction between age and sex for anisotropy (Table 14). No other difference was found between males and females by age group. We also sorted adults by age group by sex via BLR. For the young adults, 53.3% (10 of 15) of the males and 83.3% of the females were properly classified. For the middle adults, 83.3% (11 of 13) of the males and 40% (2 of 5) of the females were classified correctly. The old adult sample size was low, and that group's best BLR included only anisotropy. Nonetheless, all four males and two of the three females could be properly classified (Tables 14–17).
| Models | P (M) | P (M|data) | BFM | BF10 | Error % |
|---|---|---|---|---|---|
| Null model | 0.20 | 0.19 | 0.96 | 1.00 | |
| Sex ✻adult age group | 0.20 | 0.68 | 8.67 | 3.53 | 1.26 |
| Adult age group | 0.20 | 0.05 | 0.23 | 0.28 | 9.10 × 10−3 |
| Sex | 0.20 | 0.05 | 0.22 | 0.27 | 9.61 × 10−3 |
| Observed | Predicted | ||||
|---|---|---|---|---|---|
| Sex | Percentage correct | ||||
| Male | Female | ||||
| Age 16–35 | Sex | Male | 8 | 7 | 53.3 |
| Female | 3 | 15 | 83.3 | ||
| Overall % correct | 69.7 | ||||
| Observed | Predicted | ||||
|---|---|---|---|---|---|
| Sex | Percentage correct | ||||
| Male | Female | ||||
| Age 35–49 | Sex | Male | 11 | 2 | 84.6 |
| Female | 3 | 2 | 40.0 | ||
| Overall % correct | 72.2 | ||||
| Observed | Predicted | ||||
|---|---|---|---|---|---|
| Sex | Percentage correct | ||||
| Male | Female | ||||
| Age 50+ | Sex | Male | 4 | 0 | 100.0 |
| Female | 1 | 2 | 66.7 | ||
| Overall % correct | 85.7 | ||||
5 Discussion
Hypothesis 1 is not confirmed. The alternative hypotheses for complexity, anisotropy, RSmc, and Tfv did not exceed the plausibility of the null condition. The constancy of complexity is particularly interesting; the subadults under age 5 consumed food with the same hardness as that of older subadults, and, on average, the hardness values were moderate. Thus, most subadults consumed moderately soft diets, regardless of age. Prowse et al. (2005), Prowse et al. 2008 and Killgrove and Tykot (2013) noted that subadults often ate porridges, particularly for those not yet weaned. At Herculaneum, it is difficult to say the subadult diet is a porridge, but it lacks much in the way of hard foods.
Anisotropy is highest among the youngest individuals and drops off among the subadults in the 6–10 age group. It then increases somewhat for those age 11–15. The high anisotropy for the youngest is consistent with a limited range of jaw motion (e.g., Scott and Halcrow 2017; Kelly, Schmidt, and D'Anastasio 2020). The remainder of the subadults had anisotropy values consistent with agriculturists relying on tough terrestrial foods. The Ranked Smc and Tfv are constant across the age groups.
In terms of variation within the age groups, the subadults aged 0–5 had the lowest standard deviations for complexity and Tfv and the highest for anisotropy and Smc, while the greatest standard deviation for complexity and Tfv was in the 6–10 age group. The 6–10 age group also had the lowest standard deviations for anisotropy and RSmc (refer to Table 1). This implies the youngest subadults had the least amount of variation in terms of hardness and the most in terms of toughness. Most of the 0-5-year-olds were near the age of five, although this group included a two-year-old and a four-year old with elevated anisotropy values of 0.0067 and 0.0087, respectively. Two of the 5-year-olds had anisotropy scores above about 0.0035, which is what one would expect for people eating agricultural goods. One 5-year-old child, however, had an anisotropy of 0.0018. This individual was likely consuming a far more diverse diet than the rest of this age group.
Evidence for what dictated the subadult dietary range may be evidenced among the youngest individuals, where an important nuance emerges via the DMTA variable correlations. DMTA correlations are rare, yet in this case its presence is likely meaningful. There is a significant inverse correlation between complexity and anisotropy among the subadults in the 0–5 age group. The sample size is low, but what might be demonstrated by this relationship is a dietary dichotomy of soft/homogenous and hard/heterogeneous diets. Two of the 5-year-olds had a hard/heterogeneous diet, one 5-year-old and the 4-year-old had a soft/homogenous diet, and the 2-year-old had a hard but homogenous diet. The 2-year-old's complexity and anisotropy values could be elevated because of chewing mechanics in very young subadults (see Scott and Halcrow 2017; Kelly, Schmidt, and D'Anastasio 2020), but its combination of moderate hardness and elevated anisotropy could indicate the child was in the process of weaning.
It may well be that by the age of 5, subadults started down a certain dietary path, which they continued through the rest of their childhood; the factor determining a child's dietary path may have been their social position or socioeconomic status. Another factor may have been sex, since female social roles were rather scripted as they entered adolescence (Harlow and Laurence 2002); moreover, Avery et al. (2021) found evidence of sex-based diets emerging around age 4 to 5. However, confirmation of previous subadult sex estimates of the Herculaneum subadults has not yet been completed. In the end, what appears to be dietary homogeneity may be, in fact, an artifact of averaging at least two distinct diets, one that is more uniformly hard and one that is soft but heterogeneous.
The correlation between complexity and anisotropy ends with subadults over the age of 5 but the range of DMTA signatures jumps noticeably with the 6-10-year-olds, which has the highest complexity (3.10 in a 6-year-old) and the lowest anisotropy (0.0003) in an 8-year-old. Based on the isotopic data, Roman diets increased in meat and/or seafood as subadults aged (e.g., Prowse et al. 2004). However, for those between the ages of 6–15 at Herculaneum, their diets apparently did not include quantities of meat and/or seafood sufficient to generate a significant decline in dietary hardness. Likewise, the diet for each child over the age of 5 included about the same range of foods as subadults under age 5, hence the constancy of anisotropy. It is plausible that some of the specific foods changed as subadults aged, but the mechanical properties of those foods did not. According to Harlow and Laurence (2002), Roman subadults began to attend school (at least the boys) and take on more engaged social roles around age seven. Here again, social roles may have affected diets.
Subadults aged 6–10 had some individuals with high anisotropy values and some with low values, although the standard deviation for 6–10 year-olds was somewhat lower than that of the 0–5 year-olds. It's possible that some of the more divergent values relate to subadults having special circumstances. For example, the 8-year-old had the lowest anisotropy value of any child; it also had one of the highest complexity values. Bisel (in Capasso 2001) described this child as a “young boy with a twisted arm.” It is plausible this child had a health issue that led to a unique diet for someone of its age.
The fact that the RSmc and Tfv values remained static through childhood is not surprising. The original Smc data are strongly bimodal. Most subadults had one of two Smc values and age was not the reason for the difference. That Tfv remained constant indicates the overall ability of the diet to remove dental hard tissues did not change as subadults aged. In the context of the complexity and anisotropy data, this makes sense. It appears that subadults primarily had two dietary paths, and those paths likely remained consistent as individuals aged through subadulthood. In the end, the subadult microwear texture data mirror the isotopic data from Herculaneum in that differences among the subadults are nuanced (e.g., Martyn et al. 2018).
Hypothesis 2 is confirmed. The RSmc values are similar, but the adult Tfv mean is about 19% higher than that of the subadults. Dietary changes related to the transition to adulthood are evident. Adults had significantly lower anisotropy and higher Tfv, meaning they had a more diverse and more abrasive diet. Tfv indicates the amount of enamel surface missing due to mastication. Recall, it is not like macrowear; it is not a lifelong record of enamel loss and the higher Tfv in the adults should not be taken to indicate a longer time exposed to hard foods. Rather, it means over the several weeks before the volcanic eruption, adults were eating foods that generated deeper and/or wider micro-features compared to subadults. It could have been that they ate more food during that time, or that they ate foods that included more abrasives. Another consideration is bite force; perhaps adults chewing power led to more enamel loss (Bas et al. 2023). Bite force, however, is not strongly indicated as a source of microfeature variation because there is no difference in Tfv between 5-year-olds and 15-year-olds. Additionally, there is no difference between males and females. For these reasons, food type, rather than bite force, is considered the more likely explanation for the differences between adult and subadult microwear.
Complexity, which is often a powerful means of discerning diets in humans (e.g., El Zaatari 2008; Schmidt et al. 2016; Schmidt et al. 2019), remains constant between subadults and adults. This indicates the elements that affect dietary hardness, like the types of hard foods and the manners by which they are processed, were consistent at Herculaneum. This makes sense since all the people of Herculaneum died on the same day. Unlike the majority of other archaeological skeletal populations, at Herculaneum there is no “noise” in the DMTA data due to temporal changes in the foods chosen for consumption or seasonal variation.
The binary logistic regression sorted subadults and adults reasonably well; nearly all the adults sorted properly as did just under half of the subadults. Of those subadults classified as subadult, however, a few came from each subadult age group and most subadults had an adult-like diet. These findings affirm that there was no single subadult diet; some ate a diverse diet that was similar to that of the adults while others consumed a more homogenous diet. Likewise, there was no single adult diet, although, on average, it was more diverse compared to the subadults.
Hypothesis 3 is confirmed. The male and female anisotropy values differed when controlling for age. As females got older, their anisotropy increased. For males, it decreased. In fact, the relationship between age and anisotropy in females was among the strongest in this study. The high anisotropy in older females indicates a similar jaw motion and a more homogenous diet, compared to that of younger females and males. Female complexity is not significantly different than that of the males, indicating females ate foods about as hard as what males ate. The female average complexity, however, is elevated by relatively few hard food eaters and most females have complexity that is lower than that of the males. In fact, complexity contributes to distinguishing males and females via DFA and BLR. If adult complexity scores are ranked, eight of the top 10 values are males, including the top seven. Although males enjoyed greater social status in Roman society, harder foods are not necessarily high-status foods. In fact, most of the foods associated with high status are traditionally thought to generate only modest dental microwear like wine, high trophic level fish, and olives/olive oil. However, recent research regarding dental erosion, which pertains to changes to enamel surfaces from chemical agents, like acidic drinks, indicates that consuming fats and acids can exacerbate enamel microwear (e.g., Hara et al. 2016; Ranjitkar et al. 2017, Krueger et al. 2021). In their study, Ranjitkar et al. (2017) founded erosive surfaces led to elevated complexity values. If these findings hold up in ancient groups, it is plausible that some of the higher complexity values in the males was driven by erosion caused by the consumption of erosive products.
Male anisotropy decreased with age, which was anticipated based on the isotope research (e.g., Prowse et al. 2005; Martyn et al. 2018). Unexpectedly, the females diet contracted as they aged. Martyn et al. (2018) found that males at Herculaneum consumed more high trophic level fish. Females, on the other hand, consumed a higher percentage of terrestrial foods. If older females consumed a higher quantity of tougher foods like meat, in place of high trophic level fish, they would generate higher anisotropy values. Of course, they could still be consuming low trophic level fish, like those used to produce garum. Prowse et al. (2005) point out that those eating more marine resources also ate more olives and drank more wine. This could explain their static complexity.
The isotope data indicate both males and females ate more types of foods as they aged, although this is more strongly driven by the males at Herculaneum (Martyn et al. 2018). As males aged, their foods diverged in terms of their mechanical properties. However, the mechanical properties of female diets converged. Recall that males did not sort well by age; fewer than half clustered via DFA. As they aged, males ate more foods of differing mechanical properties than did the women. The narrowing female diet made it so that just over two-thirds of the women clustered via DFA. This is not to say the older female diet was completely homogenous; but compared to males it was less inclusive. Thus, while the isotope data point to an expanding diet for aging adults, the DMTA indicate the male and female diets changed in different ways. Figure 10 provides a boxplot that indicates the patterning of complexity and anisotropy across the adult age groups. For complexity, variation declines from the younger to the older for both males and females. For anisotropy, there is a decrease in the male values and an increase in the female values.
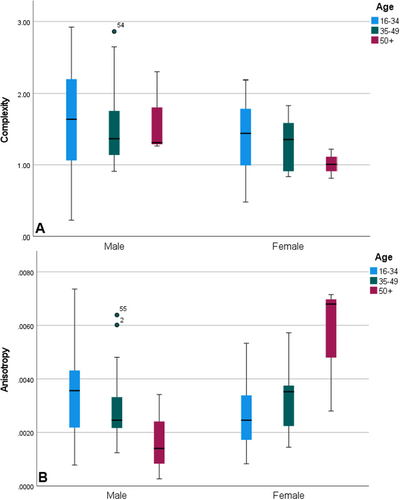
Explanations for the DMTA-indicated differences in the male and female diet include differences in food availability and preferences among the adults of Herculaneum, and the specific cultural/socioeconomic and/or health circumstances of the older people. For example, looking only at the three women from Herculaneum aged over 50 years, two had high anisotropy and one had low anisotropy like that of the males. The woman classified as male via LBR had extensive dental caries and antemortem tooth loss (Capasso 2001). Her oral health likely affected her dental microwear texture. It may be that skeletal conditions beyond oral pathology covary with diet. For example, some of the males with high complexity values had skeletal evidence of demanding physical work, which could indicate a modest labor-based social standing. If that is the case, it could be that social standing affected food access. It could also be that males were exposed to more wind-borne dust and grit, which is known to wear teeth (e.g., Smith 1972). Thus, both dietary and non-dietary factors could have elevated male complexity values.
It is worth noting that oral pathology is common among the people of Herculaneum, including dental caries and antemortem tooth loss. In fact, most people had at least one carious lesion (Capasso 2001). Therefore, except in extreme instances like the woman mentioned above, it is anticipated that dental caries' role in microwear formation was modest to the extent that it was something folded into most people's lives. In general, dental microwear signatures don't change until large lesions or antemortem tooth loss mitigate or obviate occlusion (e.g., Casserly et al. 2014). In the woman mentioned above, occlusion was just starting to be affected. Of course, people with significant molar antemortem tooth loss are not suitable for microwear analysis and are excluded. It is unknown how this selection bias affects microwear-based dietary reconstructions.
The average female complexity is two tenths lower than that of the males, a difference that is appreciable and often significant (e.g., Schmidt et al. 2019). However, a significant difference in complexity with age is obviated by tremendous dietary breadth among young adult females, who had individuals eating high and low complexity diets. The decline in the range of complexity values in the older females may be attributable, in part, to there being fewer women over the age of 35 than under. It is also important to consider, as noted earlier, that factors beyond age and sex affected dietary proclivities, and one of those may be social status.
Unfortunately, little is known concerning the social stations held by each person found at Herculaneum. Based on historic demographic data, it would be expected that several were enslaved people or those who were recently freed. One might also expect to see working class people and people of wealth (De Ligt and Garnsey 2012). One possibility is that there were more high status people of one sex compared to the other, and this disparity led to differences in diet that would impact interpretations of diet made so far. Additionally, gender identity could affect our interpretations because our demographic data are skeletal morphology-based sex estimations that calculate binary (male/female) probabilities. Identification of transgender people is limited in the archaeological record because it requires culturally-informed identity information that often does not preserve with ancient remains or is going undetected/unrecognized by bioarchaeologists. At Herculaneum, indications of gender are present, but uncommon, due to the suddenness of the pyroclastic event. Clearly, more is to be learned regarding the biological and cultural heritages of the Herculaneum people and several studies are underway to help clarify relationships between diet and biocultural phenomena including dental and skeletal morphology, cranial morphometrics, and pathology (e.g., Capasso 2001; Capasso and Di Tota 1998; Carotenuto et al. 2019; D'Anastasio et al. 2013; Tanga et al. 2020; Viciano, D'Anastasio, and Capasso 2015), and (e.g., Heilmann 2021; Gregory 2021; Viciano et al. 2011).
5.1 DMTA and Stable Isotope Analysis
Regarding the DMTA data in relation to previous stable isotope studies (e.g., Martyn et al. 2018), it is interesting that both indicate dietary distinctions within the Herculaneum population, including modest variation among the children, differences between the subadults and adults, and differences between adult males and females as they age. Isotope values represent a long-term accumulation of food-derived elements being stored in bones. Dental microwear changes in a matter of weeks or months (e.g., Teaford and Oyen 1989). The agreements between these two approaches to dietary reconstruction indicate the DMTA detected nearly the same dietary patterns among the Herculaneum people as the isotopic study detected (e.g., Martyn et al. 2018). However, the DMTA also teased out dietary nuances not detectable by stable isotopes, such as the ways the subadults differed from the adults and the sex-based differences in the older adults.
An interesting point of agreement between the isotope and DMTA data concerns Herculaneum's relatively modest dependence upon marine resources. Recall the isotope data indicate most of the Herculaneum diet came from terrestrial resources, with some foods coming from the sea (which is interesting given the number of fish bones and species found in the Herculaneum sewers [Robinson and Rowan 2015]). The DMTA data support this assertion, to the extent that the microwear texture signatures are not swamped by indicators of low microwear causing foods like fish (e.g., El Zaatari 2010). If the people of Herculaneum ate primarily fish, their complexity values would have been very low, perhaps like that of the Fuegian coastal fishers (El Zaatari 2010). That was not the case.
Although this study focuses on intrasite comparisons, it is worth nothing that, overall, the people of Herculaneum have textural values like those of farming groups from across the globe. The Herculaneum complexity and anisotropy values are comparable to Late Neolithic farmers from Belgium, Bronze and Iron Age farmers from England, Gaza, and Greece, and Iron Age farmers from Nepal. The subsistence records from these populations point to cereal grain farming and animal husbandry (Van Sessen et al. 2013; Mahoney et al. 2016, 2019; Schmidt et al. 2019; Williams, Schmidt, and Droke 2020, 2022). They also are like some New World maize farmers from Mexico and the eastern woodlands of North America (Schmidt et al. 2019; Schmidt 2021). The microwear of the Herculaneum children, however, is not the same as what is reported from Franzhausen I, in Lower Austria. There, the subset of children studied for dental microwear texture had higher complexity and lower anisotropy values compared to Herculaneum (Bas et al. 2023). There are several possible explanations for this difference, including the fact that Neolithic farming and food processing was not yet up to the level of technological advancement seen among the Romans. Not surprisingly, the people of Herculaneum are dissimilar to most foragers, who tend to have complexity and anisotropy values that vary considerably by group (e.g., El Zaatari 2010; El Zaatari and Hublin 2014; Schmidt et al. 2019; Ungar, Livengood, and Crittenden 2019).
6 Conclusion
The Herculaneum people killed by the AD 79 eruption of Mt. Vesuvius provide a unique glimpse into paleodiet; every person at the site died nearly simultaneously and they each bear the record of what they ate. There is no other archaeological site like this that has been studied via DMTA. The people excavated from Herculaneum do not necessarily represent the totality of the people who lived or visited Herculaneum, nonetheless, they allow for an unprecedented opportunity to generate a dietary reconstruction of a group who died at a particular time and place. The DMTA data indicate variations in diet that correspond with life course (e.g., age and sex) and they support isotopic work that found intra-populational dietary differences at Herculaneum and other Roman sites. Additionally, DMTA detected nuances of the Herculaneum diet that clarify dietary patterns among the subadults and adults.
Herculaneum subadult dietary variation was subtle, and not dependent upon age. Yet, subadults aged 0–5 had an inverse correlation between complexity and anisotropy indicating some subadults had a hard/diverse diet while others had a soft/homogenous diet. Adult diets were significantly more diverse than those of the subadults and removed more enamel. Moreover, adult males and females had significant dietary differences, driven primarily by a decrease in dietary breadth for females as they aged. Overall, the older adults had diets distinct from the younger adults. All told, this study underscores the value of a DMTA-based dietary reconstruction in the context of life course theory and it does so with the advantage of a population that died uniquely and devoid of the analytical challenges wrought by typical archaeological cemeteries where people died over decades and at multiple times of the year.
Author Contributions
Christopher W. Schmidt: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (lead), investigation (lead), methodology (lead), project administration (lead), resources (lead), software (lead), supervision (lead), validation (lead), visualization (lead), writing – original draft (lead), writing – review and editing (lead). Ashley Remy: conceptualization (supporting), formal analysis (supporting), investigation (equal), methodology (equal), project administration (supporting), validation (supporting), writing – original draft (supporting), writing – review and editing (supporting). Ruggero D'Anastasio: conceptualization (supporting), formal analysis (supporting), investigation (supporting), project administration (supporting), resources (equal), supervision (supporting), writing – original draft (supporting), writing – review and editing (supporting).
Acknowledgments
We thank Luigi Capasso and the staff of the University Museum, Chieti for their assistance with this project. We also thank Gregory A. Reinhardt for his assistance with photography at the museum. Additionally, we appreciate Tracy Prowse for her comments on this manuscript and Stephen Spicklemire for his assistance regarding Bayesian procedures. This project was funded by a grant from the NSF (BCS 0922930).
Ethics Statement
- Provide details on how your research project was reviewed for ethical considerations. Please list the names (and project numbers if issued) of the ethical boards, governmental agencies, community organizations and leaders, or other entities that provided review and approval for your study. If no formal approval was required, please explain why.
- Did you communicate with members of the descendant communities associated with the human remains analyzed in your research? How were local communities or other stakeholders considered when planning your research? Did they approve your study? Please provide details on the individuals and organizations who provided input on your project and explain what sort of feedback or approval these individuals or organizations provided. Does your manuscript include authors from the descendant or local communities? If members of local communities were involved with the excavation and collection of materials, please describe their involvement. If it was not possible to identify descendant communities or other stakeholders, please explain why.
- If the research was conducted in a country other than the author's own, were colleagues from the country where the research was conducted included in the study design and/or as co-authors?
- If you used archival remains or osteological collections (e.g., curated by a museum, research institute, university), please explain how the material used in your study was obtained by the curatorial institution and if any permits or other documentation were obtained to excavate, import, and/or archive/curate these materials.
- If the remains were imported, please provide details including the year the remains were imported, whether permits or other documentation were obtained, and details (names of permitting entity, permit number, etc) on any permits or documentation.
- If the research involved destructive sampling, please provide a justification in relation to the hypotheses being tested, including a statement about efforts to limit damage to the remains.
- In the case of more recent human remains, how was anonymity of those individuals maintained?
- Please confirm that any images of human remains used in the manuscript are required, and that consent has been provided by the relevant institutions, organizations, or descendant communities, as appropriate.
The images are of highly magnified dental surfaces, not images of complete skeletal elements. They are necessary to convey to scholars the kinds of surfaces we used for study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All of the data used in this study are available in Supporting Information.




