At the world's edge: Reconstructing diet and geographic origins in medieval Iceland using isotope and trace element analyses
Funding information: Stable Isotope Biogeochemistry Laboratory; Háskólasjóður Eimskipafélags Íslands; Fornminjasjóður
Abstract
Objectives
A multi-isotope study was conducted on individuals buried at Skriðuklaustur monastery (AD 1493–1554) to investigate their geographic origins and dietary composition. Comparative material from individuals excavated from Skeljastaðir, an inland farm site was also analyzed.
Materials and methods
Bone collagen was extracted from 50 humans (Skriðuklaustur and Skeljastaðir) and 25 animals (Skriðuklaustur) and analyzed for δ13C, δ15N, and δ34S. Dental enamel samples from 31 individuals (Skriðuklaustur) were also analyzed for 87Sr/86Sr, δ18O, δ13C, and trace elements (Pb, Sr, Zn, Ba).
Results
The mean value determined from individuals from Skriðuklaustur (n = 36) was δ13C = −18.7 ± 0.8‰, δ15N = 12.8 ± 1.1‰, and δ34S = 9.0 ± 1.6‰, whereas at Skeljastaðir (n = 14), it was δ13C = −20.5 ± 0.8‰, δ15N = 7.8 ± 0.9‰, and δ34S = 9.4 ± 1.6‰. At Skriðuklaustur, human dental enamel samples (n = 31) provided a 87Sr/86Sr range of 0.7060–0.7088, δ18Ophosphate from 13.9 to 16.1‰ and δ13Ccarbonate from −16.6 to −12.9‰. Inferred drinking water (δ18Odw) values range from −12.3 to −8.9‰. Sr concentrations range from 25.8 to 156.7 ppm, Ba from 0.11 to 0.81 ppm, Zn from 43.8 to 145.8 ppm, and Pb from 0.13 to 9.40 ppm.
Discussion
A combination of results indicates that the people from Skriðuklaustur were born in Iceland, but some lived inland during childhood while others lived closer to the coast. Since Skriðuklaustur was a hospital, these individuals may have sought medical treatment at the monastery. The δ13C and δ15N values determined from bone collagen indicate that the people residing at Skriðuklaustur consumed a diet high in marine protein, while those residing at Skeljastaðir exhibit values more consistent with terrestrial resources.
1 INTRODUCTION
Recent excavations at the site of Skriðuklaustur (AD 1493–1554), located in an inland valley in eastern Iceland, demonstrated that this monastery functioned not only as a place of scholarly work and monastic activities, but also a place where the community could seek medical care and treatment (Figure 1). Although oral history suggests the presence of post-Reformation burials marked by gravestones at Skriðuklaustur, these graves have never been located. Radiocarbon dating and other archaeological dating methods demonstrated that the site was in use during the monastic period, specifically between AD 1493 and 1554 (Kristjánsdóttir, 2012, pp. 153–154). Historical and archaeological evidence has revealed specialized medical knowledge, surgical tools, diverse medicinal plants and herbs, and imported objects and food, indicative of extensive involvement with foreign trade and monastic networks. In 2002–2012, the skeletal remains of around 300 individuals were excavated from the site, and these presented with a vast array of pathological conditions, including infectious diseases, traumatic injuries, and congenital anomalies (Kristjánsdóttir, 2008; Kristjánsdóttir, 2011; Kristjánsdóttir, 2012; Kristjánsdóttir & Collins, 2011). Comparative material was sought from a subset of skeletons excavated from a cemetery belonging to a single, inland farm site at Skeljastaðir in the Þjórsárdalur valley in southern Iceland (Figure 1). This site is around 60 km from the coast; however, there are numerous lakes and rivers with freshwater fish in the region. The farm was occupied earlier than Skriðuklaustur, originally believed to be from around ~AD 1000 until at least AD 1104 when the enormous eruption of Hekla occurred, likely leading to the abandonment of the site (Gestsdóttir, 2014). Radiocarbon dating of a subset (n = 7) of the human skeletal remains suggested a date range between AD 890 and 1220 (Sveinbjörnsdóttir et al., 2010). However, some human activity and animal grazing persisted in the valley at least until another eruption occurred in AD 1300 (Dugmore et al., 2007). In 1939, 63 skeletons were excavated from the early Christian cemetery at Skeljastaðir, although only 56 skeletons are available for study today (Gestsdóttir, 2014; Steffensen, 1943). This site was selected due to the extensive research conducted on it (e.g., archaeology, palaeoclimatology, multiple stable isotope analyses, palaeopathology), its well-preserved material, sample size, and geographic location. Both are inland sites without direct access to coastal resources; Skeljastaðir is located in the highlands, a mostly uninhabitable volcanic desert, while Skriðuklaustur is located in a mountainous area.
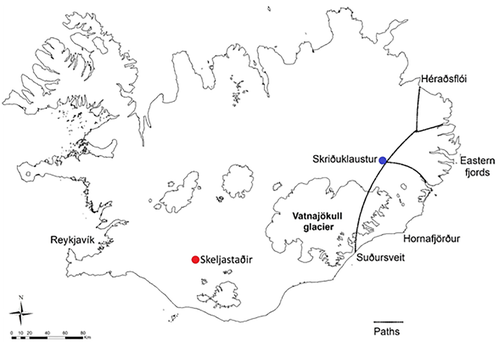
The geographic origin of the individuals buried at Skriðuklaustur is unknown; these individuals may have traveled to the monastery during pilgrimages, for trade or to seek treatment from elsewhere in the country or from abroad. The aim of this research was to undertake a multi-isotope study of human and animal remains from the site to establish the origins of the individuals buried there: did the monastery serve predominantly the local community, or had the inhabitants traveled from further afield? Considering the monastic context, this research also sought to evaluate dietary composition and the possible dietary differences between men, women, and children, or between religious and secular individuals buried at the monastery. Stable isotope analyses of δ13C, δ15N, and δ34S were obtained from samples of human and animal bone collagen and of δ13C, δ18O, and 87Sr/86Sr from human dental enamel samples. Trace element (Zn, Ba, Sr, and Pb) concentrations were also measured in the dental enamel samples to establish baselines for Icelandic humans and to attempt to further examine geographic provenance. Regarding Skeljastaðir, the previous isotopic study of δ13C and δ15N in bone collagen samples (n = 13) demonstrated a diet predominately derived from terrestrial resources (see Sveinbjörnsdóttir et al., 2010). Since δ34S values have been used in mobility and palaeodietary studies to differentiate between freshwater, marine, and terrestrial resources, we aimed to refine these interpretations by evaluating possible freshwater fish consumption at Skeljastaðir. The other previous analysis conducted on the Skeljastaðir assemblage identified only one non-Icelandic migrant according to strontium isotope analysis (see Gestsdóttir & Price, 2003). The current study investigates the utility of δ34S analyses to improve interpretations of local versus nonlocal origins within Iceland given the potential relative homogeneity of strontium isotopes deriving from the predominantly igneous basalt bedrock. Finally, we wished to compare the diet between individuals buried in these two very different settlement types (monastic and farm). Isotopic analyses have not been conducted on humans or fauna from Skriðuklaustur prior to this research and this is the first in-depth multi-isotope study of its kind undertaken on a skeletal collection dated to the Late Medieval Period from Iceland. This research thereby provides unique data that considers the link between diet and geographic provenance from an isotopic perspective. It provides previously unavailable Icelandic human and animal isotope baselines and comparative data from the Late Medieval Period that will be useful for future research concerning Iceland and other locales in the North Atlantic.
2 CURRENT EVIDENCE FOR DIET AND MOBILITY IN HISTORICAL ICELAND
Iceland is located just below the Arctic Circle. Despite the challenging, subpolar climate, people were proficient in supplying themselves with a variety of food from the beginning of the Settlement period (AD 871 ± 2) (Karlsson, 2000). During the Middle Ages, sheep, dairy products, and fish, especially dried fish, were the primary staples of the diet and a substantial proportion of terrestrial foods were acidified, soured, fermented, or salt preserved (Mehler, 2011). It was common to consume bone marrow as well, particularly from sheep long bones (Outram, 2003). Other protein sources included cow, pig, duck, goose, and various sea birds as well as eggs (Mehler, 2011; Svanberg & Ægisson, 2012). Domestic fowl (Gallus gallus sp.) have been only very rarely identified from Icelandic archaeological sites prior to the 17th century (Hamilton-Dyer, 2010). Additionally, an important trade economy was in place that provided the country with access to a variety of nonlocal food products. For example, one historical record dated AD 1567 states that for over 50 years traders from Bremen, Germany imported tons of flour, salt, beer, vinegar, grains, and bread, although most of these commodities did not become dietary staples until the 20th century (DI 15, nr. 12; Gísladóttir, 1999; Mehler, 2011). Despite ample local production, fish and meats were also imported to Iceland and exchanged for goods, such as walrus ivory and refined sulfur (Mehler, 2011).
Although barley cultivation was practiced, the growing season was short and challenging, thereby limiting the production yield (Mehler, 2011; Mooney & Guðmundsdóttir, in press; Svanberg & Ægisson, 2012). Frequent volcanic eruptions, epidemics, and the climatic changes that occurred with the Little Ice Age (~13th–19th century AD) also presented significant challenges to successful cultivation in Iceland (Dugmore & Vesteinsson, 2012; McGovern, Harrison, & Smiarowski, 2014). While some scholars suggest that plant-based foods were a minor dietary component (McGovern et al., 2014; Mehler, 2011), subsistence gardening may have been more important to the historical diet than previously believed (Kristjánsdóttir, Larsson, & Åsen, 2014). In the monastic gardens, in addition to the import and cultivation of barley, a wide variety of plants, such as nettles (Urtica dioica), angelica (Angelica archangelica), field garlic (Allium oleraceum), valerian (Valeriana officinalis), yarrow (Achillea millefolium), and greater plantain (Plantago major), were grown for dietary, utility, and medicinal purposes (Kristjánsdóttir et al., 2014; Larsson & Lundquist, 2010). According to the Sagas, ancient Icelanders even brewed mead and beer using imported malt and local barley (Gísladóttir, 1994, p. 124; Mehler, 2011) and wine was produced from foraged crowberries (Jóhannesson, 2014, p. 307). Around 30 taxa of foraged wild plants also played a small, but important role as dietary resources, particularly in times of hardship (Svanberg & Ægisson, 2012) (see Table 1).
| Common name | Icelandic name | Scientific name | Plant part | Purpose |
|---|---|---|---|---|
| Common silverweed | Tágamura | Potentilla anserine | Root | Subsistence |
| Common horsetail | Klóelfting | Equisetum arvense | Root | Subsistence |
| Garden angelica | Ætihvönn | Angelica archangelica | Root, leaves | Subsistence, medicinal |
| Scurvy grass | Skarfakál | Cochlearia officinalis | Root, leaves | Subsistence, medicinal |
| Common sorrel | Túnsúra | Rumex acetosa | Leaves | Subsistence |
| Iceland moss | Fjallagrös | Cetraria islandica | Moss | Subsistence, medicinal |
| Bilberry | Aðalbláberjalyng | Vaccinium myrtillus | Berries | Subsistence, seasoning |
| Bog bilberry | Bláberjalyng | Vaccinium uliginosum | Berries | Subsistence, seasoning |
| Crowberry | Krækilyng | Empetrum nigrum | Berries | Subsistence, seasoning |
| Dulse | Söl | Palmaria palmata | Seaweed | Subsistence |
| Carrageen moss | Fjörugrös | Chondrus crispus | Seaweed | Subsistence |
| Wild thyme | Blóðberg | Thymus praecox | Leaves, flowers | Tea, seasoning |
| Caraway | Kúmen | Carum carvi | Seeds | Seasoning |
| Common juniper | Einir | Juniperus communis | Berries | Seasoning |
| Common butterwort | Lyfjagras | Pinguicula vulgaris | Leaves | Seasoning, medicinal |
| Mountain avens | Holtasóley | Dryas octopetala | Leaves, flowers | Tea |
People residing in high latitude regions generally attain much of their protein from animal sources (Cordain et al., 2000). For example, isotopic studies conducted on the Greenland Norse revealed that in the early period of settlement approximately 20% of the diet was marine-based, increasing to 50–80% in later periods (Arneborg et al., 1999; Nelson, Heinemeier, Lynnerup, Sveinbjörnsdóttir, & Arneborg, 2012). A study of proto-Inuit individuals revealed δ15N values reached as high as 20‰ due to the frequent consumption of whales and seals (Coltrain, Hayes, & O'Rourke, 2004). Seals were a notable meat and fat source in historical Iceland, but whales were only consumed if stranded or beached (Mehler, 2011; Outram, 2003; Riddell, 2015).
In Iceland, historical and archaeological evidence suggests that subsidiary traditions primarily came from Scandinavia; however, the longstanding tradition of gathering and consuming seaweed (Dulse) likely came from Celtic settlers during the Settlement Period (Sigurðsson, 1988). Isotopic studies have demonstrated that the consumption of seaweed can raise δ13C values, resulting in a dietary signature that appears to be more marine-based (Schulting, Vaiglova, Crozier, & Reimer, 2017). Marine isotope signals could also be introduced in humans who consume terrestrial animals that graze upon seaweed (Schulting & Richards, 2009). In historical Iceland, sheep that were kept near seaweed beaches regularly grazed upon seaweeds and farmers also deliberately fed dried seaweed to their sheep for up to 18 weeks a year (Hallsson, 1964). Fishmeal, made of Icelandic herring, is often mixed with hay to supplement protein for sheep, particularly ewes (Sveinbjörnsson & Einarsson, 1998). Although it is not clear when the use of fishmeal as sheep fodder began, the steady exploitation of herring started as early as the 9th century AD (McGovern, Perdikaris, Einarsson, & Sidell, 2006). The heavy dietary reliance on meat from fish-eating sheep in historical Iceland must therefore be considered as a potential confounding factor in isotopic signals determined from human bone collagen.
2.1 Dietary reconstruction in Iceland: Carbon (δ13Ccarbonate and δ13Ccollagen), nitrogen (δ15Ncollagen), and sulfur (δ34Scollagen) isotopes
Pagan and early Christian bone samples selected from all over Iceland by Sveinbjörnsdóttir et al. (2010) produced δ15Ncollagen values between 6.5 and 15.5‰ and δ13Ccollagen values as ranging between −20.3 and −16.4‰, with most of the data in the range of −20 to −18‰. Recently, Price and Gestsdóttir (2018) reported mean δ13Ccollagen values (n = 37) as −20‰ and mean δ15Ncollagen values as 10‰, while Sayle et al. (2016) reported δ34S values between 5.5 and 14.9‰ in human samples from northern Iceland. Price and Gestsdóttir (2018) measured δ13Ccarbonate isotopes from enamel samples (n = 116) from pagan and early Christian graves throughout Iceland and obtained a mean δ13Ccarbonate value of −15.3‰ ± 0.95 (range of −17.3 to −12.4‰). Previous isotopic studies conducted on Icelandic material demonstrated overlaps between δ13C values in freshwater and marine biota, as well as between δ15N values of terrestrial herbivores and freshwater fish species. As a result, it is often difficult to differentiate between these dietary components in Iceland using δ13C and δ15N alone (Ascough et al., 2014). δ34S values, however, have been successfully used to differentiate between dietary components in Icelandic animal and human remains once local baselines are established. Sayle et al. (2016) noted in their study at Mývatn in northern Iceland that higher δ13C and δ34S values indicate marine protein as a component of regular diet, while higher δ13C and lower δ34S values indicate freshwater protein. The study also noted that higher δ13C and δ15N versus lower δ34S values indicates the consumption of a large proportion of freshwater protein, while lower δ13C and δ15N versus higher δ34S values may indicate migration from another place, likely a coastal area where δ34S values are altered by the sea spray effect (Sayle et al., 2016). This combination of isotopic analyses enabled the researchers to differentiate between terrestrial, freshwater, and marine dietary signals as well as to identify possible migrants and examine aspects of livestock trade and animal husbandry (see Sayle et al., 2016). However, due to differences in geology and volcanic activity, it must be noted that δ34S values measured in skeletal material from northern Iceland may not necessarily entirely correspond with δ34S values determined from skeletal material from southern Iceland. Isotopic value ranges established from Icelandic material (i.e., animals, water, and geology) to date are presented in Table 2.
| Material | δ13C‰ | δ15N‰ | δ34S‰ | Source |
|---|---|---|---|---|
| Birds | −23.6 to −6.9 | −4.9 to 16.4 | −5.3 to 13.6 | Ascough et al. (2014) and Wang and Wooller (2006) |
| Freshwater fish | −16.0 to −7.9 | 3.1 to 8.5 | −4.3 to −0.2 | Ascough et al. (2014) and Sayle et al. (2016)a |
| Aquatic plants | −16.9 to −11.5 | −16.0 to 4.3 | — | Ascough et al. (2014) and Wang and Wooller (2006) |
| Terrestrial plants | −30.9 to −20.4 | −12.4 to 6.5 | — | Ascough et al. (2014); Skrzypek, Paul, and Wojtun (2008); and Wang and Wooller (2006) |
| Terrestrial herbivores | −22.5 to −20.3 | −1.5 to 5.9 | −1.0 to 13.9 | Ascough et al. (2014) and Sayle et al. (2013) |
| Marine fish/mammals | −16.3 to −13.5 | 12.1 to 14.5 | 12.4 to 17.5 | Sayle et al. (2013) |
| Freshwater and geology | — | — | −2.0 to 10.0 | Sayle et al. (2013)b |
| Seawater | — | — | Mean 20.3 | Nehlich (2015) |
2.2 Residential mobility in Iceland: Strontium (87Sr/86Sr) and oxygen (δ18O) analysis of dental enamel
Price and Gestsdóttir (2006) analyzed strontium isotope (87Sr/86Sr) ratios in dental enamel from 83 pre-Christian individuals buried across the country, identifying at least 32 nonlocal migrants, that is, those having 87Sr/86Sr values above ~0.7092 - the value for rain and seawater - which defines the upper 87Sr/86Sr end member for the basaltic biosphere of Iceland. Recently, Price and Gestsdóttir (2018) expanded this study by bringing the total sample size to 127 individuals and by analyzing 87Sr/86Sr, δ18O, and δ13C from dental enamel and δ13C and δ15N from bone collagen. Icelandic inhabitants 87Sr/86Sr ratios range between 0.7055 and 0.7092 (Price & Gestsdóttir, 2018). Icelandic geologic and bioavailable strontium isotope baselines (87Sr/86Sr) are presented in Table 3. Price et al. (2015) noted that bioavailable strontium isotope ratios in Iceland are higher than those from whole rock (~0.7030–0.7037; Sigmarsson et al., 1992) due to the sea spray that occurs over much of the country. Such a phenomenon has been observed in other basaltic and particularly high-rainfall environments such as the Isle of Skye (Evans, Montgomery, & Wildman, 2009) and Hawaii (Capo, Stewart, & Chadwick, 1998). The swamping of geological 87Sr/86Sr by atmospheric deposition via rainwater and marine seasplash and spray has also been observed in maritime granitic and sandstone island biospheres such as the Western and Northern Isles of Scotland where the geology contributes 87Sr/86Sr values considerably higher than 0.7092 (Montgomery et al., 2014; Montgomery, Evans, & Cooper, 2007). Price et al. (2015) and Price and Gestsdóttir (2006, 2018) also suggested that variations in 87Sr/86Sr may reflect dietary differences between inland (values closer to 0.7030 reflecting the basalt geology) and coastal (values closer to 0.7092 reflecting the input of seawater) dwelling individuals. Strontium isotope ratios close to those of seawater in archaeological coastal and island populations are often coupled with unusually high strontium concentrations and such a combination may be explained by the high concentration of diet-derived strontium from marine plant-based resources and the extent of aerial sea spray deposition (Montgomery et al., 2007; Montgomery & Evans, 2006). Cultural culinary and husbandry practices, such as using seaweed for food, fodder, or fertilizer, grazing animals on coastal floodplains or preserving food in sea salt, may thus elevate animal and human strontium concentrations and provide a dietary 87Sr/86Sr value of 0.7092 even when humans are not consuming fish or marine mammal meat which are both low in bioaccessible strontium (unless bones are consumed) compared to plant-based foods (Montgomery et al., 2007; Montgomery & Evans, 2006).
| Enamel sample | 87Sr/86Sr | n | Source | Material | 87Sr/86Sr | Source |
|---|---|---|---|---|---|---|
| Archaeological cattle | 0.7042 | 2 | Price et al. (2015) | Bedrock | 0.7030–0.7037 | Price et al. (2015) and Sigmarsson, Condomines, and Fourcade (1992) |
| Archaeological pig | 0.7042 | 1 | Price et al. (2015) | Grass (volcanic soil) | 0.7030–0.7040 | Åberg (1995) |
| Modern redshank bird | 0.7057 | 5 | Evans and Bullman (2009) | Barley | 0.7068 | Price et al. (2015) |
| Modern sheep | 0.7059–0.7069 | 5 | Price and Gestsdóttir (2006) | Seawater | 0.7092 | Åberg (1995) |
| Modern reindeer | 0.7060 | 1 | Åberg (1995) | Rainwater | 0.7090 | Åberg (1995) |
The first published oxygen isotope ratios were measured from a small subset (n = 5) of the same Viking Age samples subjected to strontium isotope analysis by Price and Gestsdóttir (2006) (see Gestsdóttir & Price, 2006). The combined 87Sr/86Sr and δ18O values of these individuals indicate nonlocal provenance. Since then, Price and Gestsdóttir (2018) reported a mean δ18Ocarbonate value of −4.86‰ ± 0.97 (range −6.9 to −2.2‰) measured from 117 individuals from pagan and early Christian Icelandic graves. The study suggests that Icelandic δ18Ocarbonate values generally range between −7.0 and −4.0‰, with some individuals presenting with higher values (Price & Gestsdóttir, 2018). Additionally, bone and tooth samples of one early Settlement Period female migrant, Bláklædda Konan (LKS 1), were subjected to 87Sr/86Sr, δ18O, δ15N, and δ13C isotope analyses, as well as lead (Pb) and strontium (Sr) trace element analyses (Montgomery & Jakob, 2015). The results of both studies provide a limited pool of comparative data. Modern precipitation of δ18O values in Iceland ranges from −13 to −8‰ (see Price et al., 2015, figure 20; Bowen, 2018) and in modern Icelandic groundwater from −8.8 to −8.2‰ (n = 11) (Friedrich & Schlosser, 2013).
2.3 Trace element analysis of humans in Iceland: Zinc (Zn), lead (Pb), barium (Ba), and strontium (Sr)
Assessing trace element variability between individuals within or at differing archaeological sites can indicate intrapopulation and interpopulation differences in geographic provenance, particularly when the elements are nonessential and not subject to homeostatic control. These applications are made possible because living organisms absorb elements through the consumption of water and food (Jaouen & Pons, 2017). No previous trace element analyses (i.e., Zn, Pb, Ba, and Sr) have been published on archaeological Icelandic human dental enamel samples prior to this study and thus there is no comparative contemporaneous context for this data. There is the possibility that results of such analyses can be used to aid in the assessment of geographic provenance of individuals excavated from Icelandic archaeological sites, particularly for those for which a link between human elemental levels and the geographical or cultural environment can be demonstrated (Burton, Price, Cahue, & Wright, 2003; Montgomery et al., 2014). These elements have been measured; however, in Icelandic geology, groundwater, soil, and plants (see Table 4). For example, Panek and Kepinska (2002) found no evidence for anthropogenic lead input in Icelandic soil and plants, particularly in comparison with the concentrations found in Sweden and Poland.
3 METHODS
In this study, bone samples from 50 humans (36 from Skriðuklaustur and 14 from Skeljastaðir) and 25 animals (from Skriðuklaustur) were subjected to isotope analysis for carbon (δ13C), nitrogen (δ15N) and sulfur (δ34S). The sample selection was informed by state of preservation, overall skeletal completeness, age, sex, and pathologies. Osteological and pathological analyses were conducted according to standard anthropological methods (e.g., Aufderheide & Rodriguez-Martin, 2011; Brothwell, 1981; Buikstra & Ubelaker, 1994; Mitchell & Brickley, 2017; Ortner, 2003; Roberts & Connell, 2004; White, Black, & Folkens, 2011). The samples were selected from skeletal elements that did not present with pathological bone changes and were predominately taken from ribs, which record diet in the last few years of life. A previous study had undertaken dietary isotope analysis of 13 individuals from Skeljastaðir (Sveinbjörnsdóttir et al., 2010) and three of these individuals were reanalyzed here to control for interlaboratory differences. Unfortunately, no animal bones are preserved from Skeljastaðir. From Skriðuklaustur, bones samples representing a variety of animal species were sampled, including Bos taurus sp. (cattle), Capra hircus sp. or Ovis aries sp. (sheep or goat), Equus sp. (horse), Canidae sp. (dog or fox), Phocidae sp. (seal), Cygnus cygnus (swan), and marine fish, due to their differences in dietary resources and animal–human interactions. The bone collagen was extracted using a modified Longin method (Longin, 1971; O'Connell & Hedges, 1999) and the δ13C and δ15N stable isotope analyses were performed using a Costech elemental analyzer (ECS 4010) connected to a Thermo Delta V Advantage isotope ratio mass spectrometer. Finally, δ34S stable isotope analysis was performed using a Costech elemental analyzer (ECS 4010) connected to a Thermo Scientific Delta V Plus isotope ratio mass spectrometer (see Supplementary Materials). International standards, blanks and internal standards are routinely checked. Detailed analytical methods are provided in the Supplementary Materials.
Dental enamel samples from 31 of the same individuals sampled from Skriðuklaustur were also subjected to isotope analysis for strontium (87Sr/86Sr), oxygen (δ18O), and trace elements, including lead (Pb), strontium (Sr), zinc (Zn), and barium (Ba). Dental enamel samples were not selected from the remaining 19 individuals included in the bone collagen analysis due to various issues, including preservation, availability of material and ethical concerns over destructive analysis. The enamel samples were primarily selected from premolars, in which the enamel mineralizes within approximately 3 years at sometime between 2.5 and 8.5 years of age (AlQahtani, Hector, & Liversridge, 2010). In three individuals, only the third molars were available for sampling, the enamel of which mineralizes within about 4 years between 7.5 and 16.6 years of age (AlQahtani et al., 2010). Dental enamel samples were not sampled from Skeljastaðir because results from a previous study by Price and Gestsdóttir (2006) were available. The enamel samples were prepared following the procedure given in Montgomery (2002). All isotope and trace element concentrations were determined in the Department of Earth Sciences, Durham University. Carbon (δ13C) and oxygen (δ18O) isotope ratios were measured in the carbonate (CO3) component of tooth enamel by Thermo Fisher Scientific MAT 253 gas source mass spectrometer for isotope analysis. The 87Sr/86Sr ratios were determined using a Neptune multicollector inductively coupled plasma mass spectrometer (ICP-MS). Finally, enamel samples were analyzed for Sr, Ba, Zn, and Pb by ICP-MS (Thermo Scientific XSeries2) and the final enamel concentrations were then determined based on sample weights and total dilution volumes. Detailed analytical methods are provided in the Supplementary Materials.
4 RESULTS
4.1 Carbon, nitrogen, and sulfur isotope analysis in bone collagen
All human and animal skeletal samples provided well preserved bone collagen with C:N atomic ratios (SKR C:N atomic mean of 3.2, ÞSK C:N atomic mean of 3.3) falling between 3.0 and 3.4 (see Ambrose, 1990; DeNiro 1985). No notable differences were observed between sex and age groups. However, two male individuals from Skriðuklaustur (SKR 135, lower, and SKR 172, higher) and one male from Skeljastaðir (ÞSK 44, higher) had outlying δ34S values. Descriptive statistics are presented in Table 5. Overall, at Skriðuklaustur (n = 36), the range and overall δ13C mean value indicate a marine dietary signal (closer to the marine dietary end member of −12.5‰) (see Table 5 and Figure 2), while at Skeljastaðir (see Table 5 and Figure 2) (n = 14), a terrestrial dietary signal (closer to the terrestrial dietary end member of −21‰) is indicated, based upon the values adopted by Arneborg et al. (1999) according to studies conducted on individuals from Norway, Canada, western Greenland and Sweden (see Sveinbjörnsdóttir et al., 2010). The δ13C and δ15N values determined by Sveinbjörnsdóttir et al. (2010) from Skeljastaðir are included in the means, bringing the total number of individuals to n = 24 (excluding δ34S values). The δ13C sample values and mean reported by Sveinbjörnsdóttir et al. (2010) are between 1 and 2‰ higher than those determined in this study. A δ13C offset of 1–2‰ was noted even among three samples that were reanalyzed during this research (ÞSK 16, 34 and 48) despite the very similar δ15N values reported in both studies. Therefore, the overall δ13C mean reported in the present analysis at Skeljastaðir is raised by approximately 1‰ when the two datasets are combined and calculated together. This offset in the stable isotope results is difficult to explain but may be due to differences in procedures in extraction methods, stable isotope analytical protocols, and instrumentation. Overall, the carbon and nitrogen isotope ratios in adult bone collagen from Skriðuklaustur show a diet dominated by mixed marine and C3 terrestrial protein resources, which is significantly different to the predominately C3 terrestrial protein diet seen at Skeljastaðir.
| Skriðuklaustur | Skeljastaðir | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | n | δ13C‰ | δ15N‰ | δ34S‰ | Sex | n | δ13C‰ | δ15N‰ | δ34S‰ |
| Males | 12 | −18.5 ± 0.8 | 13.0 ± 1.3 | 9.2 ± 2.0 | Males | 13 | −20.4 ± 0.7 | 7.1 ± 2.1 | 9.4 ± 1.7 |
| Females | 18 | −18.8 ± 0.7 | 12.6 ± 1.1 | 9.0 ± 1.4 | Females | 11 | −20.6 ± 0.9 | 7.8 ± 0.9 | 9.4 ± 1.6 |
| Nonadults | 6 | −19.0 ± 1.2 | 12.8 ± 2.5 | 8.6 ± 1.2 | All individuals | 24 | −20.5 ± 0.8 | 7.8 ± 0.9 | 9.4 ± 1.6 |
| All adults | 30 | −18.7 ± 0.7 | 12.7 ± 1.1 | 9.0 ± 1.7 | |||||
| All individuals | 36 | −18.7 ± 0.8 | 12.8 ± 1.1 | 9.0 ± 1.6 | |||||
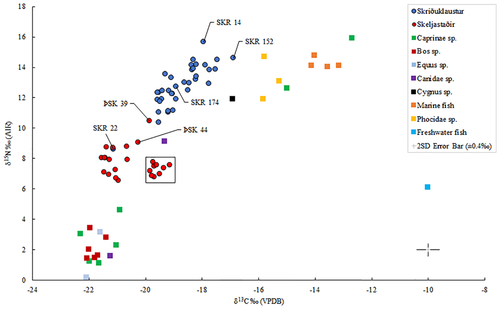
The mean values of δ13C, δ15N, and δ34S determined in animal bones (n = 25) from Skriðuklaustur are presented in Table 6 and plotted in Figures 1 and 2. The isotope values determined in the human bone samples can only be compared with animal baselines from Skriðuklaustur (Figure 2 and Table 6) and elsewhere in Iceland (see Table 2). The Cygnus sp. (swan) sample had relatively high δ13C (−16.9‰) and δ15N (11.9‰) values. While swans are generally herbivorous, they are known to incidentally consume the tiny animals (e.g., worms, fish, molluscs) that are often found in the weeds that they eat. The Bos sp. (cow) and Equus sp. (horse) samples showed δ13C and δ15N values consistent with herbivorous diets. Meanwhile, Phocidae sp. (seals) and saltwater fish samples had δ13C and δ15N values consistent with diets predominately derived from marine sources. Among Canidae species, one sample had a very low δ15N value of 1.6‰, while the other had a significantly higher δ15N value of 9.1‰. This difference may suggest that these two samples represent different species (e.g., domestic dog and wild arctic fox) or the same species with differing diets. Additionally, two Caprinae sp. (sheep/goat) samples, which must be domestic, displayed notably higher carbon and nitrogen isotope ratios (δ13C ~ −13.8‰, δ15N ~ 14.3‰, δ34S ~ 13.4‰) than the others (n = 5) (mean δ13C −21.6 ± 0.5‰, δ15N 2.5 ± 1.3‰, and δ34S 4.1 ± 1.6‰), indicating a diet substantially derived from marine resources (see Schulting et al., 2017) (see Figures 2 and 3a). For comparison, Sayle et al. (2013) reported sheep/goat samples with means of δ13C −21.2 ± 0.4‰, δ15N 2.5 ± 1.1‰, and δ34S 6.7 ± 1.9‰ but did not report any outliers in δ15N values. This provides evidence that notably different husbandry feeding practices were being followed for domestic animals.
| Common name | Species | n | Range δ13C‰ | Mean δ13C‰ | Range δ15N‰ | Mean δ15N‰ | Range δ34S‰ | Mean δ34S‰ |
|---|---|---|---|---|---|---|---|---|
| Goat/sheep | Caprinae sp. | 7 | −22.3 to −12.7 | −19.4 ± 0.9 | 1.1–15.9 | 5.8 ± 5.5 | 2.1 to 15.2 | 6.8 ± 4.5 |
| Cow | Bos sp. | 6 | −22.1 to −21.4 | −21.8 ± 0.2 | 1.4–3.4 | 2.1 ± 0.7 | 5.4 to 9.6 | 7.0 ± 1.3 |
| Horse | Equus sp. | 2 | −22.1 to −21.6 | ~−21.8 | 0.2–3.2 | ~1.7 | 2.2–9.0 | ~5.6 |
| Dog or fox | Canidae sp. | 2 | −21.2 to −19.3 | ~−20.3 | 1.6 to 9.1 | ~ 5.4 | 2.5–5.7 | ~4.1 |
| Swan | Cygnus sp. | 1 | — | −16.9 | — | 11.91 | — | 6.1 |
| Seal | Phocidae sp. | 3 | −15.8 to −15.3 | −15.6 ± 0.2 | 11.9–14.7 | 13.2 ± 1.1 | 10.7 to 12.0 | 11.4 ± 0.5 |
| Freshwater fish | — | 12 | −11.4 to −9.1 | −9.8 ± 0.6 | 5.0–6.8 | 5.9 ± 0.6 | −4.2 to −0.2 | −2.7 ± 1.4 |
| Saltwater fish | — | 4 | −14.0 to −13.2 | −13.7 ± 0.4 | 14.0–14.8 | 14.3 ± 0.3 | 12.9–14.4 | 13.7 ± 0.6 |
| Herbivores | — | 15 | −22.3 to −12.7 | −20.7 ± 2.7 | 0.17–15.9 | 3.8 ± 4.3 | 2.1–15.2 | 6.9 ± 3.5 |
4.2 Strontium, oxygen, and carbon isotope and trace element analysis in dental enamel
The δ18O, δ13C, and 87Sr/86Sr (n = 31) determined from the dental enamel samples are presented in Table 7 and isotope ratio means and ranges are presented in Table 8. The humans from Skriðuklaustur range from 0.7060 to 0.7088 and all fall between the lower geological end member of the basalt and the upper end member for the Icelandic biosphere of rain and seawater, that is, 0.7030–0.7092. All the humans and animals are therefore consistent with origins in Iceland or other regions of basaltic or marine limestones, the only two types of rocks which are known to produce biosphere values below 0.7092. The human δ18Ocarbonate values range from 22.8 to 25.0‰, mean = 24.2 ± 0.6‰. Using the equations provided in Chenery, Pashley, Lamb, Sloane, and Evans (2012) (δ18Ophosphate = 1.0322 × δ18Ocarbonate − 9.6849 and δ18Odrinkingwater = 1.590 × δ18Ocarbonate[VSMOW] − 48.634), this equates to a δ18Ophosphate range of 13.9–16.1‰ and mean of 15.3 ± 0.6‰. The δ18Odrinkingwater values range from −12.3 to −8.9‰. Converting enamel δ18O to precipitation significantly increases the uncertainty on individual measurements, which according to Chenery et al. (2012) is ±1‰ (2 SD). Nonetheless, such a range for human enamel fits within the annual δ18O range for modern precipitation in Iceland (−13 to −8‰) (see Price et al., 2015, figure 20; Bowen, 2018) and is close to values obtained from modern groundwater (range −8.8 to −8.2‰; n = 11) (Friedrich & Schlosser, 2013). It is possible that changes in past climate such as the Little Ice Age may have an impact on the values of contemporaneous drinking water sources in the North Atlantic region (Daux, Lécuyer, Adam, Martineau, & Vimeux, 2005; Fricke, O'Neil, & Lynnerup, 1995) but these results define a δ18Odw range in excess of 3‰ at −12.3 to −8.9 ± 1‰ (2 SD) for medieval humans who appear to be of Icelandic origin with regards to their strontium isotope ratios. The δ13Ccarbonate values, which derive from whole diet during childhood, range from −16.6 to −12.9‰ with a mean of −15.2 ± 0.8‰. All individuals, aside from SKR 10 and SKR 14, fell within the expected range of a diet primarily derived from C3 terrestrial resources (−17.0 to −14.0‰; see Kellner & Schoeninger, 2007; Froehle, Kellner, & Schoeninger, 2012; Neil, Montgomery, Evans, Cook, & Scarre, 2017).
| Sample | Tooth | Bone | Sex | Age | 87Sr/86Sr | δ18Op‰ | δ18Odw‰ | δ13Ccarb‰ | δ13Cco‰ | δ15Nco‰ | δ34Sco‰ | Sr ppm | Pb ppm | Zn ppm | Ba ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SKR 4 | LPM2 max | Rib | M | OA | 0.70706 | 13.9 | −12.3 | −15.4 | −19.6 | 11.9 | 9.5 | 47.84 | 9.60 | 115.94 | 0.27 |
| SKR 10 | RM3 max | Rib | F | OA | 0.70874 | 14.4 | −11.5 | −13.2 | −19.2 | 11.0 | 8.26 | 156.68 | 0.30 | 145.83 | 0.51 |
| SKR 14 | RPM1 mand | Rib | — | NA | 0.70887 | 15.6 | −9.7 | −12.9 | −17.9 | 15.7 | 10.72 | 140.41 | 0.58 | 119.40 | 0.25 |
| SKR 22 | LPM1 mand | Rib | — | NA | 0.70746 | 15.7 | −9.5 | −15.3 | −21.1 | 8.6 | 7.10 | 46.04 | 2.65 | 112.90 | 0.11 |
| SKR 23 | RPM2 mand | Rib | F | YA | 0.70798 | 15.8 | −9.4 | −15.3 | −19.2 | 11.1 | 10.43 | 72.39 | 4.08 | 90.91 | 0.80 |
| SKR 29 | RPM1 mand | Rib | F | YA | 0.70753 | 14.7 | −11.1 | −15.5 | −19.2 | 12.5 | 8.63 | 40.80 | 2.28 | 73.51 | 0.41 |
| SKR 30 | — | Rib | F | OA | — | — | — | — | −19.5 | 12.3 | 11.23 | — | — | — | — |
| SKR 33 | LPM2 mand | Rib | F | OA | 0.70859 | 15.9 | −9.3 | −14.7 | −18.2 | 14.2 | 9.64 | 97.45 | 1.69 | 90.85 | 0.43 |
| SKR 46 | LPM2 mand | Rib | — | NA | 0.70845 | 15.9 | −9.3 | −14.8 | −19.1 | 12.3 | 9.38 | 88.18 | 2.73 | 101.84 | 0.17 |
| SKR 65 | LPM2 max | Rib | F | YA | 0.70742 | 13.8 | −12.5 | −16.4 | −19.1 | 13.3 | 9.75 | 44.14 | 3.51 | 66.99 | 0.81 |
| SKR 81 | LPM2 mand | Rib | F | YA | 0.70757 | 16.0 | −9.1 | −15.7 | −19.4 | 11.9 | 6.28 | 45.15 | 0.73 | 70.79 | 0.69 |
| SKR 91 | LPM2 mand | Rib | M | YA | 0.70790 | 15.6 | −9.7 | −15.1 | −19.1 | 12.3 | 9.26 | 52.90 | 0.53 | 91.57 | 0.17 |
| SKR 100 | LPM2 mand | Rib | M | YA | 0.70834 | 15.2 | −10.3 | −14.5 | −18.9 | 11.9 | 11.58 | 93.70 | 2.68 | 104.60 | 0.40 |
| SKR 115 | LPM1 mand | Rib | M | OA | 0.70686 | 15.9 | −9.3 | −15.7 | −18.2 | 13.2 | 8.11 | 71.20 | 0.35 | 95.30 | 0.60 |
| SKR 122 | LPM2 max | Parietal | F | YA | 0.70747 | 14.4 | −11.6 | −15.5 | −19.3 | 13.5 | 9.49 | 44.40 | 1.38 | 134.59 | 0.62 |
| SKR 126 | LPM2 max | Rib | F | OA | 0.70781 | 15.6 | −9.7 | −15.2 | −18.6 | 13.0 | 8.67 | 67.25 | 0.51 | 126.08 | 0.63 |
| SKR 128 | LPM2 max | Rib | F | OA | 0.70813 | 14.9 | −10.8 | −16.0 | −17.5 | 13.9 | 8.61 | 64.13 | 0.51 | 87.03 | 0.29 |
| SKR 130 | — | Rib | M | OA | — | — | — | — | −17.5 | 14.5 | 8.52 | — | — | — | — |
| SKR 135 | LM3 max | Rib | M | YA | 0.70727 | 16.1 | −8.9 | −15.2 | −18.3 | 13.8 | 12.86 | 45.83 | 0.64 | 73.10 | 0.72 |
| SKR 144 | — | Rib | F | YA | — | — | — | — | −18.5 | 13.0 | 8.01 | — | — | — | — |
| SKR 146 | — | Rib | — | NA | — | — | — | — | −18.3 | 14.5 | 8.70 | — | — | — | — |
| SKR 150 | RPM2 mand | Rib | M | YA | 0.70726 | 14.4 | −11.5 | −16.1 | −18.4 | 14.2 | 8.54 | 43.69 | 0.39 | 47.30 | 0.46 |
| SKR 152 | LPM2 mand | Rib | M | YA | 0.70832 | 15.6 | −9.7 | −14.5 | −16.9 | 14.6 | 11.06 | 53.13 | 0.23 | 112.00 | 0.27 |
| SKR 155 | LM3 max | Rib | M | OA | 0.70840 | 15.4 | −10.0 | −14.5 | −18.5 | 12.5 | 9.05 | 73.54 | 0.21 | 123.89 | 0.29 |
| SKR 163 | — | Rib | — | NA | — | — | — | — | −18.3 | 13.9 | 7.95 | — | — | — | — |
| SKR 167 | RPM1 mand | Rib | M | YA | 0.70723 | 14.4 | −11.5 | −16.6 | −19.5 | 10.4 | 7.08 | 18.31 | 0.26 | 67.54 | 0.17 |
| SKR 169 | RPM1 max | Rib | F | OA | 0.70809 | 15.5 | −9.9 | −14.9 | −19.0 | 11.2 | 10.57 | 82.28 | 0.33 | 70.56 | 0.70 |
| SKR 172 | RPM1 max | Rib | M | OA | 0.70684 | 15.2 | −10.4 | −15.1 | −17.7 | 13.8 | 5.10 | 43.18 | 0.34 | 81.92 | 0.13 |
| SKR 174 | RC max | Femur | M | OA | 0.70602 | 16.0 | −9.1 | −15.5 | −18.9 | 12.7 | — | 74.97 | 1.46 | 104.97 | 0.61 |
| SKR 181 | LPM2 max | Temporal | F | OA | 0.70857 | 16.0 | −9.1 | −14.5 | −19.4 | 12.4 | 6.81 | 79.55 | 0.41 | 116.02 | 0.18 |
| SKR 189 | LPM1 mand | Rib | F | YA | 0.70700 | 15.1 | −10.4 | −15.8 | −19.6 | 12.3 | 9.47 | 43.90 | 9.40 | 145.50 | 0.40 |
| SKR 195 | LPM2 mand | Rib | F | YA | 0.70874 | 15.6 | −9.7 | −14.0 | −17.9 | 14.1 | 9.28 | 111.26 | 0.45 | 43.76 | 0.51 |
| SKR 201 | LPM1 max | Rib | F | YA | 0.70816 | 15.1 | −10.4 | −16.3 | −18.2 | 13.4 | 8.54 | 80.90 | 1.36 | 141.54 | 0.23 |
| SKR 221 | RPM2 mand | Rib | — | NA | 0.70767 | 15.5 | −9.8 | −15.3 | −19.5 | 11.8 | 7.84 | 25.79 | 0.13 | 98.61 | 0.22 |
| SKR 226 | RPM1 mand | Rib | F | OA | 0.70778 | 15.4 | −10.1 | −15.1 | −19.5 | 11.1 | 6.48 | 87.33 | 0.61 | 75.60 | 0.46 |
| SKR 241 | LPM1 mand | Rib | F | OA | 0.70800 | 15.3 | −10.1 | −15.4 | −17.8 | 12.9 | 11.43 | 50.39 | 3.12 | 88.33 | 0.30 |
| ÞSK 2 | ? | ? | F | OA | 0.70905 | — | — | — | −19.7 | 7.5 | — | — | — | — | — |
| ÞSK 3 | — | Rib | F | OA | — | — | — | — | −21.0 | 6.6 | 8.5 | — | — | — | — |
| ÞSK 4 | — | Rib | F | YA | — | — | — | — | −20.7 | 8.8 | 7.7 | — | — | — | — |
| ÞSK 5 | ? | ? | F | YA | 0.70591 | — | — | — | −19.8 | 7.2 | — | — | — | — | — |
| ÞSK 12 | ? | ? | F | OA | 0.70562 | — | — | — | −19.2 | 7.6 | — | — | — | — | — |
| ÞSK 15 | ? | ? | F | OA | 0.70615 | — | — | — | −19.6 | 7.6 | — | — | — | — | — |
| ÞSK 16 | ? | Rib | F | OA | 0.70754 | — | — | — | −21.0 | 6.7 | 11.5 | — | — | — | — |
| ÞSK 17 | ? | Rib | F | OA | 0.70662 | — | — | — | −21.2 | 8.7 | 9.6 | — | — | — | — |
| ÞSK 26 | ? | ? | M | OA | 0.70610 | — | — | — | −19.7 | 6.8 | — | — | — | — | — |
| ÞSK 29 | — | Rib | M | OA | — | — | — | — | −21.5 | 7.1 | 9.5 | — | — | — | — |
| ÞSK 32 | ? | Rib | M | YA | 0.70685 | — | — | — | −21.4 | 8.8 | 8.1 | — | — | — | — |
| ÞSK 34 | — | Rib | M | YA | — | — | — | — | −20.3 | 9.1 | 8.8 | — | — | — | — |
| ÞSK 37 | ? | Rib | M | OA | 0.70900 | — | — | — | −20.6 | 7.9 | 9.1 | — | — | — | — |
| ÞSK 38 | ? | ? | M | OA | 0.70608 | — | — | — | −19.5 | 7.0 | — | — | — | — | — |
| ÞSK 39 | ? | ? | F | OA | 0.70972 | — | — | — | −19.9 | 10.5 | — | — | — | — | — |
| ÞSK 41a | ? | ? | M | YA | 0.70704 | — | — | — | −19.7 | 7.8 | — | — | — | — | — |
| ÞSK 41b | — | Rib | M | OA | — | — | — | — | −21.3 | 7.9 | 8.2 | — | — | — | — |
| ÞSK 42 | ? | Rib | M | OA | 0.70653 | — | — | — | −21.4 | 8.0 | 8.6 | — | — | — | — |
| ÞSK 44 | — | Fibula | M | OA | — | — | — | — | −21.6 | 8.1 | 13.7 | — | — | — | — |
| ÞSK 47 | ? | ? | M | YA | 0.70682 | — | — | — | −19.8 | 6.9 | — | — | — | — | — |
| ÞSK 48 | ? | Rib | M | OA | 0.70614 | — | — | — | −21.4 | 8.0 | 9.2 | — | — | — | — |
| ÞSK 51 | — | Rib | F | OA | — | — | — | — | −21.1 | 7.3 | 11.4 | — | — | — | — |
| ÞSK 54 | — | Rib | F | YA | — | — | — | — | −21.3 | 7.0 | 7.7 | — | — | — | — |
| ÞSK 60 | ? | ? | M | OA | 0.70718 | — | — | — | −19.4 | 7.4 | — | — | — | — | — |
- Abbreviations: δ18Op, δ18Ophosphate VSMOW (‰); δ18Odw, δ18Odrinkingwater VSMOW (‰); δ13Ccarb, δ13Ccarbonate VPDB (‰); δ13Cco, δ13Ccollagen (‰); δ15Nco, δ15Ncollagen (‰); δ34Sco, δ34Scollagen (‰); ÞSK, Skeljastaðir; F, female; LPM1, left first premolar; LPM2, left second premolar; LM3, left third molar; M, male; mand, mandible; max, maxilla; NA, nonadult (<17); OA, older adult (36+); RC, right canine, RPM1, right first premolar; RPM2, right second premolar; RM3, right third molar; SKR, Skriðuklaustur; Sr/Pb/Zn/Ba ppm, strontium/lead/zinc/barium parts per million; YA, young adult (17–36).
| Samples | n | Lead (Pb) ppm | Barium (Ba) ppm | Zinc (Zn) ppm | Strontium (Sr) ppm |
|---|---|---|---|---|---|
| Male | 11 | 0.39 | 0.29 | 95.3 | 52.9 |
| Female | 16 | 1.05 | 0.49 | 89.59 | 69.82 |
| Nonadult | 4 | 1.62 | 0.20 | 107.37 | 67.11 |
| All | 31 | 0.61 | 0.40 | 95.3 | 64.13 |
| Ranges (all) | 31 | 0.13–9.40 ppm | 0.11–0.81 ppm | 43.76–145.83 ppm | 25.79–156.68 ppm |
| Samples | n | 87Sr/86Sr | δ18Ophosphate‰ (VSMOW) | δ18Odrinkingwater‰ (VSMOW) | δ13Ccarbonate‰ (VPDB) |
|---|---|---|---|---|---|
| Male | 11 | 0.70741 ± 0.00144 (2 SD) | 15.2 ± 0.7 | −10.2 ± 1.1 | −15.3 ± 0.6 |
| Female | 16 | 0.70797 ± 0.00099 (2 SD) | 15.2 ± 0.6 | −10.3 ± 1.0 | −15.2 ± 0.8 |
| Nonadult | 4 | 0.70811 ± 0.00114 (2 SD) | 15.7 ± 0.1 | −9.6 ± 0.2 | −14.6 ± 1.0 |
| All | 31 | 0.70779 ± 0.00132 (2 SD) | 15.3 ± 0.6 | −10.2 ± 1.0 | −15.2 ± 0.8 |
| Ranges (all) | 31 | 0.70602–0.70887 | 13.8–16.1 | −12.3 to −8.3 | −16.6 to −12.9 |
The trace element (Ba, Zn, Pb, and Sr) concentrations (n = 31) determined from the dental enamel samples are presented in Table 7 and Supplementary Figures S1 and S2 and the medians and ranges in Table 8. Trace element bioavailability is affected by the complex interaction of numerous variables that occur during mineral metabolism. Strontium is a nonessential trace element that enters biological tissues via calcium pathways following the ingestion and metabolization of food and drink. In omnivores, such as humans, known antagonisms, and synergisms with other elements and food types result in skeletal strontium being predominately derived from the plant part of the diet. It passively enters and remains in the skeleton by substituting for calcium and is not subject to homeostatic control. The amount of strontium incorporated into skeletal tissues is dependent on dosage but is thought to reflect the amount of strontium that is bioavailable from the local environment, diet, dietary calcium, and any strontium released from the skeleton through calcium homeostasis (Montgomery, Evans, Chenery, Pashley, & Killgrove, 2010). Similarly, barium is a sensitive dietary indicator that, like strontium, is not subject to homeostatic control but is absorbed by plants in smaller amounts and may be discriminated according to rising trophic chain position (Szostek, Głąb, & Pudło, 2009). In this study, the strontium concentrations range from 25.8 to 156.7 ppm with a median of 64.1 ppm and the barium concentrations determined in the same individuals range from 0.11 to 0.81 ppm with a median of 0.40 ppm. Ancient and modern dental enamel concentrations of zinc reportedly range from 58 to 2,100 ppm according to a combination of studies reviewed by Ezzo (1994). Some studies have identified a positive correlation between zinc and lead concentrations and areas of increased urbanization and industrialization (see Ezzo, 1994; Tvinnereim, Eide, Riise, Fosse, & Wesenberg, 1999). Lead concentrations in dental enamel as associated with geological or environmental exposure, rather than from anthropogenic exposure, are generally less than ~0.7 ppm (Millard et al., 2014; Montgomery et al., 2010). The zinc concentrations in this study range from 43.8 to 145.8 ppm with a median of 95.3 ppm, while the lead concentrations range from 0.13 to 9.4 ppm with a median of 0.61 ppm.
5 DISCUSSION
5.1 Monasteries, travel, and trade
Medieval Icelandic monasteries were normally located on major travel routes or near coastal settlements where most of the population resided at the time. Although Skriðuklaustur now appears to be situated in a remote inland valley, during its occupation it was centrally located on a major routeway between the northern and southern parts of the country (Kristjánsdóttir, 2012, p. 296; Kristjánsdóttir, 2016). Until the 17th century, when the route closed due to climate change, pilgrims, patients, fish traders and other individuals easily traveled over the Vatnajökull glacier to reach Skriðuklaustur in the Fljótsdalur valley (Björnsson, 2009, p. 243; Kristjánsdóttir, 2016) (Figure 1). Severe environmental and epidemiological conditions were likely to have been major catalysts for the movement of people to Skriðuklaustur, which represents one of the largest skeletal assemblages excavated in Iceland and a high prevalence of pathological conditions. The Black Death first came to Iceland at the beginning of the 15th century, killing more than half of the population (Karlsson, 2000, pp. 114–117; Kristjánsdóttir, 2016; Júlíusson, 2018). The second wave of the Plague occurred around AD 1495–1496, just after the establishment of monastery (Kristjánsdóttir, 2016). Furthermore, the only confidently diagnosed cases of treponemal disease in Iceland were found at Skriðuklaustur (Kristjánsdóttir, 2012; Walser, Kristjánsdóttir, Gowland, & Desnica, 2018), indicating that immediately following the plague, an outbreak of treponemal disease occurred in Iceland contemporaneously with the European epidemic of the late 15th century (see Walker, Power, Connell, & Redfern, 2014). The Black Death and other plagues in Iceland also coincided with climate changes such as sustained summer rains and cooling weather, which lead to grass and crop failure, increased disease burden, caused food shortages and increased the number of homeless people (Kristjánsdóttir, 2016).
It is known that the brethren residing at Skriðuklaustur aimed to buy farms near the coast: access to the valuable resources (e.g., fish, driftwood, whales, and seals) that coastal sites offered being the probable driver. Those running monasteries found a variety of ways for earning money, including participation in local and international trade (Steinsson, 1965, p. 108; Steinsson, 1966; Kristjánsdóttir, 2016). For example, refined sulfur, an important commodity in medieval trade was found at Skriðuklaustur (Kristjánsdóttir, 2012; Mehler, 2011), possibly for medicinal uses or the production of vermilion (Mehler, 2015). Other notable imports include an effigy of Saint Barbara, a monastic trumpet and rare ceramic pottery imported from France (Kristjánsdóttir, 2012; Mehler, Kristjánsdóttir, & Kluttig-Altmann, 2018). Other sources of income included donations from benefactors from the local community, the sale of books and payment for medicinal treatment and community charity (Kristjánsdóttir, 2016). The Skriðuklaustur monastery also partly depended upon foreign commerce for dietary resources. A large amount of fish bones, primarily from cod (Gadus morhua), ling (Molva molva), haddock (Melanogranmus aeglefinus), shark, and rays were also found during the excavation at Skriðuklaustur, indicating that marine fish were indeed an important dietary component. Smaller fish (60–80 cm in length) are generally found in the Greenland Sea and around the northern and eastern coasts of Iceland, while larger fish (often over 100 cm), such as those found at Skriðuklaustur, are normally caught around the southern and western coasts (Kristjánsdóttir, 2016). It is important to note that larger fish tend to reach higher trophic levels and elevated isotope values (Schoeninger & DeNiro, 1984; Häberle et al., 2016). Fish were both dietarily and culturally important at Skriðuklaustur, where religious fasting was practiced (Kristjánsdóttir, 2017). Zooarchaeological research demonstrated that fresh fish were regularly consumed at the monastery unlike at most inland sites where only dried fish are normally found (Hamilton-Dyer, 2010; Pálsdóttir, 2006). This notable difference was undoubtedly connected with the religious fasting practiced at the monastery (Pálsdóttir, 2006). However, bipedal animals such as poultry were also permitted for consumption during fasting periods among some Augustinian and other monastic orders (Kristjánsdóttir, 2017) and seals were also still consumed during the fast.
The results of bone collagen stable isotope analyses for carbon and nitrogen on individuals from Skriðuklaustur align with the historical and zooarchaeological evidence, demonstrating a diet substantially derived from marine and potentially freshwater fish protein in some individuals (see Figure 2). No sex-based differences in dietary intake were noted. Based on a trophic shift of +1‰ for carbon and 5.5 ± 0.5‰ for nitrogen (see Fernandes, 2015; Sayle et al., 2016) resulting in a δ13C value of −20.7‰ and a δ15N value of 7.7 ± 0.5‰, none of the individuals at Skriðuklaustur subsisted on an entirely terrestrial protein diet in adulthood. However, if the two sheep that grazed on seaweed and/or fishmeal (see Figure 2) are included, then a + 5.5 ± 0.5‰ trophic shift results in a δ15N value of 9.2 ± 0.5‰. In this case, one individual (SKR 22; 16.5–18.5 years old) with a significantly lower δ15N value (8.6‰) consumed a solely terrestrial diet. Since the individual (SKR 22) exhibits a cleft lip (cleft premaxilla) and palate (cleft maxilla) (Barnes, 2012) (Figure 4) and a differential diagnosis of treponemal disease based on gummatous cranial and tibial lesions (see Aufderheide & Rodriguez-Martin, 2011; Hackett, 1976; Ortner, 2003), poor overall health and dietary restrictions are suggested. Palatal clefts or perforations can occur due to several reasons, including late stage syphilis, and often cause significant difficulties in eating, drinking and speaking (Ilczuk-Rypuła, Pietraszewska, Kempa, Zalewska-Ziob, & Wiczkowski, 2017; Patil, 2016). Additionally, individuals born with cleft palate generally have difficulties breastfeeding and take longer to adapt to eating solid foods than other children (Müldner et al., 2009; Wiet, Biavati, & Rocha-Worley, 2017). Similarly, Müldner et al. (2009) noted a high-status individual with cleft palate from Whithorn Cathedral priory, Scotland (13th–14th century) that also exhibited δ13C and δ15N values consistent with a predominately terrestrial diet, unlike the mixed marine and terrestrial diet shown by the other bishops and clerics buried at the priory. SKR 22 contrasts with SKR 14, another nonadult who appears to have been one of the highest marine protein consumers throughout life.
5.2 Mobility and geographic provenance
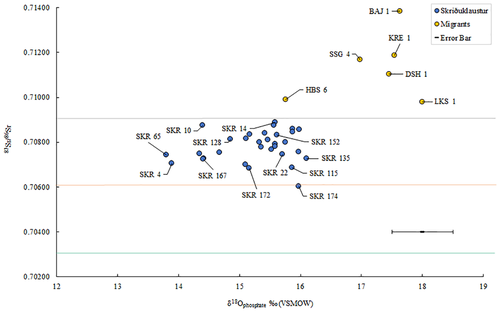
Prior to this research, the origin of the individuals buried at Skriðuklaustur was unknown. Due to the site's hospital, monastic, and trade network functions, it was hypothesized that the individuals buried there could be represented by locals, foreign traders, pilgrims or patients seeking treatment from elsewhere in the country or from abroad (Kristjánsdóttir, 2012; Kristjánsdóttir, 2017). The strontium and oxygen isotope results demonstrate that all the individuals sampled during this study were likely to be of indigenous origin (see Table 7 and Figure 5). None of the individuals exceed the 87Sr/86Sr value of rain and seawater (0.7092), which implies an Icelandic origin. The possibility that some individuals had originally resided on chalk, which has a range of 0.708–0.709, (e.g., Denmark and Southern Britain) cannot be completely ruled out (see Evans, Chenery, & Montgomery, 2012; Evans, Montgomery, Wildman, & Boulton, 2010; Montgomery et al., 2014). However, the δ18Ophosphate values are inconsistent with an origin in Britain/Ireland, where δ18Ophosphate would have to fall within the range of 16.3–19.1‰ (see Montgomery et al., 2014). Furthermore, the δ18Ophosphate range is ~2‰, which is normal for a single temporally contemporaneous population, indicating that these individuals most likely represent a local group of people. Overall, the results suggest that the individuals sampled from the temporally constrained (1493–1554 AD) cemetery at Skriðuklaustur were of Icelandic origin. As the 87Sr/86Sr value approaches the value of rain and/or seawater, δ13Ccarbonate values move away from a wholly terrestrial value thereby suggesting a higher input of marine-derived strontium and carbon into the diet (Figure 6). However, it is important to note that δ13Ccarbonate reflects whole diet (e.g., fat, carbohydrates, and protein) rather than just the protein component of the diet. Nonetheless, the variability in the plot of δ13Ccarbonate and 87Sr/86Sr may indicate that some individuals (e.g., SKR 167) consumed a wholly terrestrial diet while living further from the coast (lower δ13Ccarbonate, more basaltic-derived 87Sr/86Sr values) during childhood, while others with higher δ13Ccarbonate and more marine-derived 87Sr/86Sr values (e.g., SKR 10 and 14) appear to have consumed marine resources and lived near the coast where sea spray and splash were more prevalent. This positive correlation is strengthened by the Sr concentrations which are also positively correlated with δ13Ccarbonate and 87Sr/86Sr: as Sr isotope ratios approach the seawater value the amount of Sr in the enamel increases (see Figure 6). This suggests that the higher values are indicative of individuals that grew up in a coastal area where the food web was impacted by marine sea spray and splash, consumption of seaweed or seaweed-eating fauna, while those with lower ratios and concentrations resided further inland where the basalt dominated the food chain. The correlation between these three parameters is logical but rarely observed so clearly in human populations—highlighting the advantages of studying populations inhabiting the geologically homogenous islands providing two-end member systems—and suggests that it is possible to use isotope analysis to identify residence within different residential zones in Iceland.
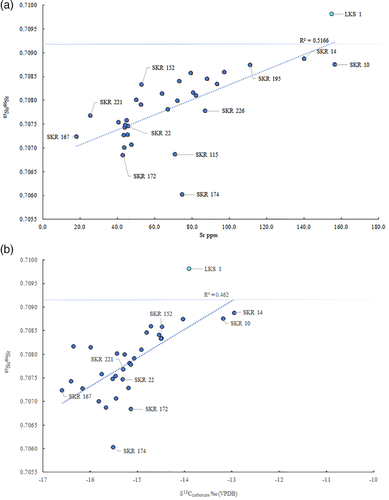

One older adult male (SKR 174) with bone changes indicative of Paget's disease (e.g., expansion of cranial diploë, endocranial “cotton wool” appearance and diffuse, irregular new bone formation throughout the cranium and all long bones) (see Ortner, 2003) had the lowest 87Sr/86Sr value, which may imply that he resided at a site further inland during childhood (see Figure 7). According to clinical research, individuals with Paget's disease involving the tibia, femur, or acetabular portion of the ilium have clinically and statistically significant functional and mobility impairments (Lyles et al., 1995). Aside from limited mobility, the condition often causes muscular diseases (e.g., atrophy) and sensory or psychological impairments (e.g., hearing loss, dementia) (Kimonis et al., 2008; Monsell, 2004) that can lead to social disability (see Roberts, 2000). This individual possibly moved to Skriðuklaustur from a site further inland for treatment or hospice care, potentially with the aid of his community. According to written documents, medieval Icelandic monasteries each served specific regions or districts of the country, meaning that people traveled to their regional monastery when in need of their services (Kristjánsdóttir, 2016; Kristjánsdóttir, 2017). In some cases, bodies were even moved from the place of death to the local monastery for proper burial and funerary services (Kristjánsdóttir, 2017, pp. 134, 248–249). While not all monasteries in Iceland served as hospitals, they were generally obligated to provide hospitality to travelers and the poor (Kristjánsdóttir, 2016; Kristjánsdóttir, 2017). As Skriðuklaustur served the south-eastern area of the country (Kristjánsdóttir, 2016) (see Figure 7), the isotope results might suggest that some of the sampled individuals migrated there from other places within its district. On the other hand, according to the 87Sr/86Sr results of Price and Gestsdóttir (2006), only one individual (ÞSK 39) out of 33 analyzed from Skeljastaðir was of nonlocal origin. This individual also exhibits a δ15N value significantly higher than other individuals measured from Skeljastaðir both in this study and as reported in Sveinbjörnsdóttir et al. (2010) (see Figure 2). The difference in this individual's diet may therefore be correlated with their foreign provenance prior to their migration to Skeljastaðir.

5.3 Dietary reconstruction
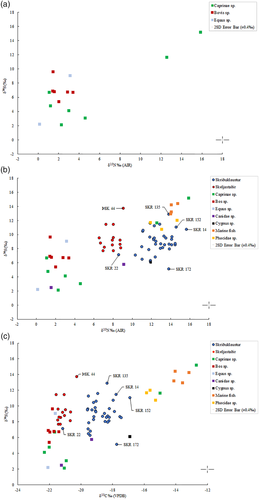
The results of this study indicate that a primarily terrestrial protein diet was consumed at Skeljastaðir, thus confirming the results by Sveinbjörnsdóttir et al. (2010). Within a few decades of AD 1000, during the Medieval Warm Period, sea fishing in the North Atlantic increased dramatically (Barrett, Locker, & Roberts, 2004). The lack of significant marine dietary input at Skeljastaðir may possibly relate to a reduced reliance on sea fishing during the occupation of the site (1000–1104 AD). However, this may also simply relate to the long distance (~60 km) between Skeljastaðir and the coast. In parallel, using δ13C and δ15N isotope analysis on British skeletons, Müldner and Richards (2007) demonstrated that High Medieval populations consumed significantly more marine resources than populations from earlier periods and that fasting regulations imposed by the church at this time were the probable cause. Although there is no documentary evidence regarding Skeljastaðir, it is archeologically well known that up to 15 other farms successfully operated in the Þjórsárdalur valley prior to the AD 1104 eruption of Hekla. The region has ample grazing land and bodies of water filled with freshwater fish (Dugmore et al., 2007; Gestsdóttir, 2014; Steffensen, 1943; Þórðarson, 1943). When compared with the δ13C and δ15N values from bone collagen, the results of the sulfur isotope (δ34S) analysis suggest that some individuals at Skeljastaðir were consuming more freshwater protein than others, while those from Skriðuklaustur appear to have been consuming both saltwater and freshwater resources (see Table 7 and Figure 3b,c). At Skriðuklaustur, one individual (SKR 172) exhibits a high δ13C and δ15N with a notably lower δ34S value, indicating a diet with freshwater protein and possibly movement from an area further inland (see Figure 3b,c). Three individuals (SKR 14, 135, 152) show higher δ13C, δ15N, and δ34S values suggesting a diet high in marine protein or movement from a coastal area. Overall, the human δ34S values for both sites define a similar range and are consistent with 87Sr/86Sr values, indicating a population strongly impacted by marine sulfur. Furthermore, SKR 14, an adolescent, presented with a δ13Ccarbonate value of −12.9‰ and δ13Ccollagen value of −17.9‰ as well as the highest δ15Ncollagen value (15.7‰) among the individuals sampled from Skriðuklaustur, likewise indicating a high dependency upon marine dietary resources. Similarly, one young adult male (SKR 152) appears to have maintained a marine dietary signal since childhood, exhibiting high δ13Ccarbonate value, the highest δ13Ccollagen value, and high δ15N and δ34S bone collagen values, implying a coastal origin. At Skeljastaðir, one male individual (ÞSK 44) showed higher δ34S and δ13C values than the others, indicating movement from a coastal area to the inland site (see Figure 3c). However, it is particularly important to consider in the context of Iceland that sulfur concentrations in flora and water sources fluctuate in response to volcanic activity (Sayle et al., 2013). One study demonstrated that due to magmatic degassing, sulfur concentrations were elevated in water sources near Skeljastaðir in the Þjórsárdalur valley even 15 years after the last eruption of Hekla (Holm et al., 2010). Although the impact of volcanic activity in the Veiðivötn–Bárðarbunga system on sulfur concentrations in flora and water near Skriðuklaustur are unknown, the severe eruption of Veiðivötn in AD 1477 caused the permanent abandonment of many of the farms in the nearby Hrafnkelsdalur valley and likely other sites in the east as well (Rafnsson, 1990, p. 93, 100). Skriðuklaustur was established just 16 years later (Kristjánsdóttir, 2016) implying that the surrounding environment may have still been considerably altered by volcanic emissions. As sulfur isotope ratios may reflect both diet and geological provenance, it is important to consider that δ34S values may increase in populations residing close to active, or erupting, volcanic systems.
5.4 Trace element analyses
The trace element analyses of dental enamel determined low Ba concentrations, which probably results from the low Ba content in seawater and Icelandic groundwater and basalt (see Naimy, 2008) (see Supplementary Figure S1). The low values, small range, and little variation in Ba concentrations also corroborate the interpretation that the people residing at Skriðuklaustur represented a local population of individuals that grew up in Iceland. The means for Pb indicate that at least some anthropogenic exposure to lead occurred at Skriðuklaustur (Supplementary Figures S1 and S2). Only 12 individuals exhibited Pb concentrations greater than ~0.7 ppm (see Table 7), which is normally considered the threshold in archaeological human dental enamel between natural and anthropogenic Pb exposure (Millard et al., 2014; Montgomery et al., 2010). All the individuals with anthropogenically elevated lead levels are nonadults or are biologically female except for the older adult male with Paget's disease (SKR 174), potentially indicating differential exposure related to gendered social roles or the life course. Lead exposure may occur from dermatological contact with lead objects or structures, inhalation of lead dust or paint chips or the ingestion of food, soil, or other substances contaminated with lead dust. Women generally spent more time indoors, tasked with maintaining the household, food preparation and weaving textiles that were used both as clothing and currency (vaðmál). Due to the importance of cloth currency for its use to pay tithes, taxes and other legal or economic transactions, cloth production was intensely standardized. Some evidence even suggests that legal controls may have regulated occupational roles and simultaneously that cloth currency production was also an important form of female agency to Icelandic society (Norrman, 2008; Smith, 2014). Additionally, the accumulation, retention, and susceptibility to the health effects of exposure to toxic metals have been demonstrated to differ between men and women, particularly during pregnancy or menopause (Vahter, Åkesson, Lidén, Ceccatelli, & Berglund, 2007). Regarding children, it is well established that they not only absorb a far greater amount of ingested lead than adults but are also more frequently exposed to it due to hand-to-mouth activities and other behavioral tendencies such as outdoor play (see Jacobs & Nevin, 2006; Wittmers, Aufderheide, Rapp, & Alich, 2002). Women and children may have been more regularly exposed to lead at home due to more frequent contact with lead objects and structures within the household. A young adult female (SKR 189) with cystic echinococcosis (hydatid disease) had a Pb concentration of 9.40 ppm, significantly higher than all others in the sample set, indicating substantial childhood anthropogenic exposure to lead (see Montgomery et al., 2010). The individual (SKR 23) with the second highest Pb concentration (4.1 ppm) was a young adult (c. 17–25) female with treponemal disease, exhibiting skeletal changes consistent with venereal syphilis, according to the criteria described by Hackett (1976) and Ortner (2003). Clinical research has also demonstrated that deficiencies in essential minerals, such as calcium, can result in the abnormal and rapid absorption of toxic heavy metals or trace elements, such as Pb, particularly in malnourished children (Talpur, Afridi, Kazi, & Talpur, 2018). It is thereby possible that some individuals with elevated lead concentrations may have had low calcium intake during childhood. It is evident that parts of the cemetery were contaminated with anthropogenic lead, which is likely to be associated with the infrastructure of the monastery (e.g., lead window frames; door hinges) and objects found on site (Kristjánsdóttir, 2012). By the 17th century, lead-glazed kitchenware significantly increased in availability in Iceland, particularly among high status individuals, but it has also been found at sites dating to the early medieval period (Þorgeirsdóttir, 2010). For context, Rasmussen, Skytte, Jensen, and Boldsen (2015) measured Pb concentrations in individuals that resided in rural, monastic, and urban sites around Denmark and northern Germany. Their results indicate that higher status, urban dwellers were more likely to live among lead structures (e.g., window frames, roof tiles possibly in contact with drinking water) and be able to afford lead or lead-glazed kitchenware. Another female (SKR 65) exhibited a Pb concentration of 3.51 ppm. She was one of a few individuals found buried within the church itself, potentially indicating that she was a benefactor or had a special status at the monastery (Kristjánsdóttir, 2010; Walser et al., 2018). It is therefore possible that the individuals with elevated anthropogenic Pb concentrations were exposed to lead within their households or the monastic grounds, if they resided there during childhood. Nonetheless, with a small number of exceptions the range of Pb concentrations determined in dental enamel is largely below ~0.7 ppm, suggesting that those analyzed from Skriðuklaustur represent individuals that grew up in an unpolluted environment, such as Iceland (see Montgomery et al., 2014).
The highest Zn concentration was 145.8 ppm (SKR 10), which is still well within the lower spectrum of expected Zn concentrations in dental enamel (9.9–1,550 ppm) (see Jaouen, Herrsher, & Balter, 2017). Clinical studies have noted a relationship between malnutrition and lower enamel zinc concentrations (Brown et al., 2004) and that enamel Zn concentrations of <90 ppm may reflect marginal zinc supply during childhood (e.g., Tvinnereim et al., 1999). The individuals with the lowest zinc concentrations include an adult female (SKR 195) (43.8 ppm) and an adult male (SKR 150) (47.3 ppm), possibly implying limited zinc supply during childhood (see Supplementary Figure S2). These two individuals also exhibit dental enamel hypoplasia, a pathological indicator of metabolic or health stress during childhood (see Ortner, 2003). However, zinc is an essential trace element under homeostatic control and its concentrations are altered by numerous and complex interactions including diet, disease, individual variation, digestion, and absorption. As a result, zinc concentrations determined in dental enamel may not accurately reflect palaeodiet (Dolphin & Goodman, 2009; Ezzo, 1994).
6 CONCLUSIONS
The skeletons sampled from Skriðuklaustur appear to represent individuals from the geographical region (south-east quarter of Iceland) served by the monastery. The 87Sr/86Sr, δ13Ccarbonate, and δ18Ophosphate and trace element concentrations (Pb, Ba, Zn, Sr) results indicate that the individuals analyzed in this study were likely to have been born in Iceland but the clear positive correlation between 87Sr/86Sr, δ13Ccarbonate, and Sr concentrations indicates that some had lived inland during childhood and some closer to or at the coast. Additionally, no significant differences between males and females were observed. There is thus no evidence that imported foodstuffs were shifting the human 87Sr/86Sr outside the Icelandic range. This research also provides the first dataset for inferred drinking water (δ18Odw) values determined from human dental enamel from Iceland. The δ18Odw values range from −12.3 to −8.9‰, with a mean of −10.2 ± 1.0‰, which fit within the annual δ18O for precipitation in Iceland (−13 to −8‰) (see Price et al., 2015, figure 20; Bowen, 2018). The enamel Ba and Pb concentrations (ppm) were low, probably due to the low concentrations of these elements in the Icelandic environment and saltwater, which may corroborate the conclusion of Icelandic origins of those buried at Skriðuklaustur. These individuals may have sought medical treatment or hospice at the monastery, as was common in Iceland at the time (see Kristjánsdóttir, 2017). Despite the over 350 year time difference, the δ13C and δ15N values determined in bone collagen indicate that the individuals residing at Skriðuklaustur consumed a diet high in marine protein, while those residing at Skeljastaðir exhibit values more consistent with reliance on terrestrial resources. No significant differences between men and women were observed at either site. When δ13C and δ15N values are compared with the δ34S values, the results indicate that three individuals from Skriðuklaustur and one individual from Skeljastaðir may have migrated from coastal sites or areas heavily affected by sea spray. The δ34S values also imply freshwater fish consumption at both sites. At least one individual from the Skriðuklaustur assemblage consumed a different diet than others residing at the monastery, potentially due to the pathological conditions they were suffering from. Three individuals from Skriðuklaustur may have experienced a period of illness or metabolic stress during childhood according to their low Zn concentrations and the presence of linear enamel hypoplasia. Finally, approximately 12 individuals from Skriðuklaustur may have been exposed to anthropogenic sources of lead during childhood (Pb concentrations >0.7 ppm), possibly from the lead objects and window frames that were found during the excavation of the monastery ruins.
Overall, the dietary differences noted between the two noncontemporary inland sites may reflect cultural changes in trade, subsistence strategies, and environment (e.g., the Little Ice Age, volcanic eruptions) in medieval Iceland. Furthermore, the results of isotope analyses conducted on individuals excavated from Skriðuklaustur indicates that the monastery was operated, visited and inhabited by the local population of brethren, pilgrims, patients, and other local individuals. Considering the functions of the monastery, these findings also provide further evidence for the movement of disease (e.g., syphilis), goods (e.g., lead wares), food (e.g., fish, fruit), and information (e.g., medicine) from other parts of Iceland and abroad, implying that these past people were never found in isolation, even at the edge of the world.
ACKNOWLEDGMENTS
The authors would like to thank the National Museum of Iceland for access to the collection, facilities, and laboratory. The authors would also like to thank Kayla Crowder, Bryony Rogers, Joanne Peterkin, and Dr Kurt Gron for assistance in the isotope laboratory and with analyses conducted at the Department of Archaeology, Durham University. This research was financed by Fornminjasjóður (the Archaeology Fund), Háskólasjóður Eimskipafélags Íslands (the Eimskip University Fund), and the Stable Isotope Biogeochemistry Laboratory (SIBL). We gratefully acknowledge a NERC Capital Call grant to Dr Gröcke (#CC018) that provided funding for the purchase of a dedicated sulphur isotope mass spectrometer in the Stable Isotope Biogeochemistry Laboratory (SIBL) at Durham University.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.




