Cranial and endocranial diversity in extant and fossil atelids (Platyrrhini: Atelidae): A geometric morphometric study
Funding information Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Fundaçao de Amparo da Pesquisa do Estado de São Paulo, Grant/Award Number: 2017/16451-2; Fondo para la Investigación Científica y Tecnológica, Grant/Award Number: PICT-2014-1810; Consejo Nacional de Investigaciones Científicas y Técnicas, Grant/Award Number: PIP-112-200901-00132; Universidad Nacional de La Plata, Grant/Award Number: PI-UNLP N787
Abstract
Objectives
Platyrrhines constitute a diverse clade, with the modern Atelidae exhibiting the most variation in cranial and endocast morphology. The processes responsible for this diversification are not well understood. Here, we present a geometric morphometric study describing variation in cranial and endocranial shape of 14 species of Alouatta, Ateles, Brachyteles, and Lagothrix and two extinct taxa, Cartelles and Caipora.
Methods
We examined cranial and endocranial shape variation among species using images reconstructed from CT scans and geometric morphometric techniques based on three-dimensional landmarks and semilandmarks. Principal components analyses were used to explore variation, including the Procrustes shape coordinates, summing the logarithm of the Centroid Size, the common allometric component, and residual shape components.
Results
Differences in endocranial shape are related to a relative increase or decrease in the volume of the neocortex region with respect to brainstem and cerebellum regions. The relative position of the brainstem varies from a posterior position in Alouatta to a more ventral position in Ateles. The shape of both the cranium and endocast of Caipora is within the observed variation of Brachyteles. Cartelles occupies the most differentiated position relative to the extant taxa, especially in regards to its endocranial shape.
Conclusions
The pattern of variation in the extant species in endocranial shape is similar to the variation observed in previous cranial studies, with Alouatta as an outlier. The similarities between Caipora and Brachyteles were unexpected and intriguing given the frugivorous adaptations inferred from the fossil's dentition. Our study shows the importance of considering both extant and fossil species when studying diversification of complex traits.
1 INTRODUCTION
The New World monkeys, or platyrrhines, constitute a very diverse clade of primates (Aristide, Rosenberger, Tejedor, & Perez, 2015; Fleagle, 2013; Rosenberger, Tejedor, Cooke, & Pekar, 2009). The three extant families (Atelidae, Cebidae, and Pitheciidae) exhibit variation in morphological traits such as body size, cranial shape, and relative brain size and shape (Allen & Kay, 2012; Aristide, dos Reis, et al., 2015 Aristide et al., 2016 Aristide, Rosenberger, et al., 2015; Hartwig, Rosenberger, Norconk, & Young Owl, 2011; Isler et al., 2008), as well as in species number and ecology (Campbell, Fuentes, MacKinnon, Bearder, & Stumpf, 2010). Neontological and paleontological studies suggest that nearly the full suite of platyrrhine diversity seen across the clade today originated relatively early in the evolutionary history of the group, during the Early Miocene (Aristide, Rosenberger, et al., 2015; Opazo, Wildman, Prychitko, Johnson, & Goodman, 2006; Perez, Tejedor, Novo, & Arístide, 2013; Rosenberger, 1992). Previous work also suggests that the diversification of platyrrhines was a relatively rapid adaptive radiation driven by several factors such as diet, body size, and social and locomotor behaviors (Aristide et al., 2016; Aristide, Rosenberger, et al., 2015; Marroig & Cheverud, 2001; Perez, Klaczko, Rocatti, & dos Reis, 2011; Rocatti, Aristide, Rosenberger, & Perez, 2017; Rosenberger et al., 2009).
Although less studied, marked variation within families in endocranial (i.e., endocast as a proxy of brain shape) and cranial size and shape is also a notable feature of platyrrhines (Aristide et al., 2016; Aristide, dos Reis, et al., 2015; Halenar, 2012; Halenar, Cooke, Rosenberger, & Rímoli, 2017; Halenar-Price & Tallman, 2019; Isler et al., 2008; Youlatos, Couette, & Halenar, 2015). Compared to the other two platyrrhine families, the Atelidae stands out as it displays the highest degree of morphological variation in cranial and endocranial characteristics (Aristide et al., 2016, 2018; Perez et al., 2011). The four extant atelid genera form a monophyletic clade that originated about 15–20 Ma ago (Aristide, Rosenberger, et al., 2015; Perez et al., 2013) and also displays a high degree of variation in diet and social organization (Rosenberger & Strier, 1989). A simplistic view of the family would place the spider monkey, Ateles, at one end of a continuum, with a large globular brain case, small face, and frugivorous dentition, and the howler monkey, Alouatta, at the other end, with a small, elongated brain case, large face, and folivorous dentition. The woolly monkey, Lagothrix, and woolly spider monkey, Brachyteles, are intermediate between these two extremes in cranial morphology and brain size and shape. Brachyteles does complicate the picture, however, as this genus has a relatively large rounded neurocranium like the one of Ateles combined with large, crested molars similar to those seen in Alouatta (Rosenberger, 1992; Rosenberger, Halenar, & Cooke, 2011; Rosenberger & Strier, 1989).
Several nearly complete and undistorted fossil crania have been attributed to the Atelidae and provide the opportunity to achieve a more profound understanding of the patterns of variation observed in the extant species. Particularly, two nearly complete fossil skeletons (Figure 1), Caipora bambuiorum (Cartelle & Hartwig, 1996), and Cartelles coimbrafilhoi (Halenar & Rosenberger, 2013; Hartwig & Cartelle, 1996), provide a wealth of anatomical information. Thought to be late Pleistocene in age, these taxa provide a window into the morphometric diversity in the clade prior to or during two key events for biodiversity changes in South America: the Pleistocene mega-faunal extinction and the human expansion into the Americas (Haynes, 2009; Koch & Barnosky, 2006). These two fossils are also of particular interest because they have been reconstructed at approximately 20–25 kg, which is about 1.5–2 times as large as the largest living platyrrhines (Cartelle & Hartwig, 1996; Halenar, 2011a; Hartwig & Cartelle, 1996).
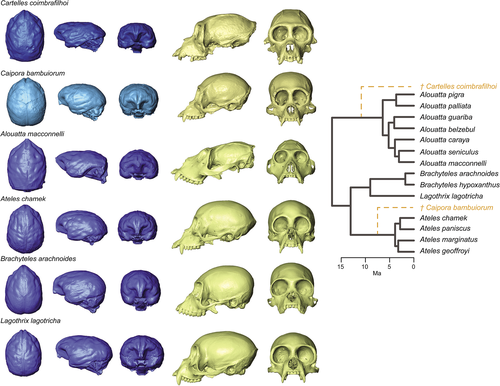
The two fossil taxa were found close to each other, both being discovered in the Toca da Boa Vista (TBV) cave complex in Bahia, Brazil. TBV is an extensive limestone travertine with over 100 km of underground galleries located in a drought-prone semiarid zone of northeastern Brazil, known as caatinga, which constitutes a savanna-like corridor of vegetation that separates the Amazon and Atlantic rainforests (Auler et al., 2004). The phylogenetic position of these taxa is controversial. The unusually robust Caipora has been considered as a close relative of spider monkeys (Ateles). Conversely, Cartelles was first included as part of the hypodigm of the fossil Protopithecus brasiliensis, and both specimens were included by some as members of the subfamily Alouattinae (Cartelle & Hartwig, 1996; Halenar & Rosenberger, 2013; Hartwig & Cartelle, 1996; Rosenberger et al., 2015). Cartelles presents a bit of an evolutionary puzzle, which requires more explanation, as its cranial morphology exhibits traits generally assumed to be derived in the howler monkey clade (i.e., an elongated and unflexed cranial base, a large airorhynchus face, a posteriorly directed nuchal plane), whereas its dentition is more primitive and shows none of the morphological characteristics related to the highly folivorous diet of this clade (Halenar & Rosenberger, 2013; Hartwig & Cartelle, 1996; Rosenberger et al., 2011, 2015).
Here, we perform an exhaustive morphometric study of variation in cranial and endocranial size and shape in Cartelles coimbrafilhoi and Caipora bambuiorum, within the context of the Atelidae as a whole. Despite the existence of these and other relatively well-preserved specimens of platyrrhine fossil crania, detailed studies of morphological variation have generally not been systematically pursued using extant and fossil species together (but see Fleagle, Gilbert, & Baden, 2016 who address this issue across primates). Specifically, the main aim of this work is to more comprehensively explore the pattern of variation in cranial and endocranial morphology in atelid primates. We explore morphometric variation in cranial and endocranial morphology by integrating three-dimensional (3D) computerized tomography images and geometric morphometrics. These techniques allow one to study features such as the relative position and the size of individual structures seen in the endocast in addition to overall absolute and relative brain size/endocranial volume. Based on previous cranial results using different configurations of data and comparative samples (i.e., Fleagle et al., 2016; Halenar, 2012; Halenar et al., 2017; Krupp, Cartelle, & Fleagle, 2012), we expect variation in cranial and endocranial size and shape in the fossil species to be within the range of that already observed in the extant family Atelidae, but not necessarily within the range of each of the extant genera.
2 MATERIALS AND METHODS
2.1 Samples
We included 14 species from Central and South America belonging to the four extant genera of atelids (Alouatta, Ateles, Brachyteles, and Lagothrix; see details in Table S1; Wilson & Reeder, 2005), as well as two Pleistocene fossil species, Cartelles coimbrafilhoi [Museu de Ciências Naturais PUC Minas Gerais, Belo Horizonte, Brazil (MCL 06)] and Caipora bambuiorum (MCL 05; Figure 1; Cartelle & Hartwig, 1996; Hartwig & Cartelle, 1996; Halenar & Rosenberger, 2013). The extant specimens are housed in the Museu de Zoologia (MZUSP, Universidade de Sao Paulo, Brazil), the Museu Nacional (MNRJ, Rio do Janeiro, Brazil), the Museo Argentino de Ciencias Naturales (MACN, Buenos Aires, Argentina), and the Smithsonian National Museum of Natural History (USNM, Washington, DC). Several specimens were downloaded from the DMM-KUPRI repository (dmm.pri.kyoto-u.ac.jp/; Table S2).
All extant specimens studied were defined as adults based on the presence of completely erupted dentition. Cartelles and Caipora meet this criterion as well; however, Caipora has been described as a “late-stage subadult” due to unfused epiphyses throughout its postcranial skeleton (Cartelle & Hartwig, 1996). Approximately, equal proportions of male and female specimens were selected for each extant species and pooled for morphometric and comparative analyses (Perez et al., 2011). A total of 50 specimens were measured for cranial analyses, whereas 49 were included in endocast measurements (Table S2). Eight individuals included in these data sets do not match, as the preservation state of some specimens precluded their inclusion in both (Table S2).
2.2 Geometric morphometrics
Specimens were scanned using either a medical CT scanner (Philips Brilliance CT 64-slice; pixel size = 0.14–0.28 mm, slice thickness = 0.33–0.45 mm, slice interval = 0.50–0.66 mm) or a table top micro-CT scanner (SkyScan/Bruker, model 1,173; pixel size and slice thickness were between 45 and 67 μm). A 3D virtual endocast was generated for each specimen from the CT or μCT scan data using ITK-SNAP 3.0 (www.itksnap.org) and following a threshold-based two-dimensional segmentation procedure (see description in Aristide, dos Reis, et al., 2015). Additionally, 3D surface models of the cranium were extracted from the tomographic data using 3DSlicer 4 (www.slicer.org) (Figure 1). All 3D models were saved in PLY file format. Given that the fossil specimens present some damage, particularly in the region of the zygomatic arches (which are completely absent in the case of Cartelles; see Figure 1), two strategies were followed to be able to analyze them without limiting the anatomical regions of the skull to be studied. In the case of Caipora, the right arch is absent, but the left side is intact. Thus, in the 3D model, we reflected the undamaged side, taking advantage of bilateral symmetry, into the damaged side. This way we obtained a virtually repaired complete specimen (Figure 1). In the case of Cartelles, given that both sides are absent, we resorted to a thin-plate spline-based imputation method, as implemented in the geomorph R package (Adams & Otarola-Castillo, 2013), to estimate the position of the missing landmarks in the damaged regions.
One of the authors (LA) digitized 26 anatomical landmarks and 373 semilandmarks along the curves and surfaces of each endocast, as well as 64 anatomical landmarks and 196 semilandmarks on each cranium (see Aristide et al., 2016, 2018 for details; Table S3 and Figure S1). The surface semilandmarks of each structure (endocast and cranium) were digitized automatically using a mesh of roughly equidistant points that makes use of the landmarks and curve semilandmarks as reference points and a thin-plate spline interpolation (Aristide et al., 2016), in the geomorph (Adams & Otarola-Castillo, 2013), and Morpho (Schlager, 2013) packages for R software (R-Development Core Team, 2017).
The landmarks and semilandmarks were aligned by means of Generalized Procrustes Analysis, which translates, scales and rotates the coordinates of landmarks and semilandmarks using a least squares algorithm (Mitteroecker & Gunz, 2009; Rohlf & Slice, 1990). The semilandmarks were also slid along the tangent direction of curves and surfaces using a bending energy criterion (Gunz, Mitteroecker, & Bookstein, 2005). The centroid size (CS) of the point configurations was used as the size measure for each structure (Mitteroecker & Gunz, 2009; Rohlf & Slice, 1990).
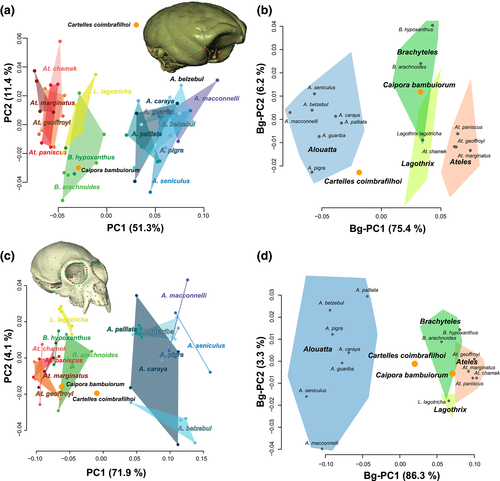
A principal component (PC) analysis was performed on the covariance matrix of all superimposed coordinates (Procrustes shape coordinates) for extant and fossil specimens to reduce the dimensionality of the shape space (shape PC; Mitteroecker & Gunz, 2009). We also performed a Between-group principal component analysis based on the covariance matrix of mean shape coordinates for the extant species. The extant and fossil individual specimens were projected onto the between-group PCs scores space (Bg-PC; Mitteroecker & Bookstein, 2011). In these Bg-PC analyses, species mean shapes for both endocast and cranium were also overlaid with a minimum spanning tree to connect the nearest neighbors in shape space, which might not always be the points closest to each other in the two dimensions visible in the PC ordination results.
The much larger body size of Cartelles and Caipora compared to the extant comparative sample means that size, as well as shape, is an important component of this investigation. Therefore, we explored variation in the size–shape space, or form variation, among extant and fossil specimens. For this purpose, we augmented the matrix of Procrustes shape coordinates by including the logarithm of the centroid size, and then performed a PC analysis on the covariance matrix of these variables (form PC; Mitteroecker, Gunz, Bernhard, Schaefer, & Bookstein, 2004). We also explored variation in the common allometric component (CAC) and residual shape components (RSCs). The CAC is the scaled vector of regression slopes estimated using a pooled regression of the shape variables on the log centroid size. The RSCs describe the nonallometric component and are the PC scores of the residuals of the pooled regression analysis (Mitteroecker et al., 2004). The surface semilandmarks, Procrustes shape coordinates, log centroid size, principal component scores, and the pooled regression variables were obtained or calculated using geomorph (Adams & Otarola-Castillo, 2013) and Morpho (Schlager, 2013) packages for R software (R-Development Core Team, 2017).
3 RESULTS
3.1 Endocranial size and shape
The PC ordination of the 14 extant and 2 extinct atelid species based on their endocast shape variation is shown in Figure 2a. Alouatta and Ateles represent extremes of variation, while Lagothrix and Brachyteles species occupy more intermediate positions in shape space, although they do fall closer to Ateles than to Alouatta (Figure 2a). With respect to the fossil species, whereas Caipora falls within the observed variation of the extant genus Brachyteles, Cartelles occupies a region in endocast shape space that does not overlap with the variation seen in any of the extant species or genera. A similar result is suggested by the ordination of the species based on the Bg-PC of shape variation (Figure 2b). However, in this analysis, Cartelles occupies a position in endocranial shape space closer to that seen in extant Alouatta, and is in fact linked to A. guariba specifically by the minimum spanning tree (Figure 2b; Figure S2a).
It is important to note that the main shape changes are concentrated on the first PC axis for both the traditional principal component (PC1: 51.3% and PC2: 11.4%; Table S4) and the between-group principal component (Bg-PC1: 75.4% and Bg-PC2: 6.2%) analyses. The differences in shape among the individuals along the first PC axis are related to the relative increase of the neocortex area with respect to the brainstem and cerebellum in the genus Ateles; and the relative position of the brainstem, together with the flexion of the basal region of the brain endocast, which varies from a posterior position in Alouatta to a more ventral position in Ateles (Figure 3a; Figure S3a). Shape variation along PC2 involves overall changes in the globularity of the endocast, mostly within each genus (Figure 3a; Figure S3a). Taking this into consideration, the endocranial shape of Cartelles is remarkable as it presents a strikingly flat and elongated appearance that explains its particular location in the atelid endocast morphospace (Figures 1, 2a,b, and 3a).
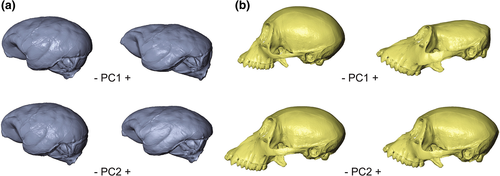
Figure 4a shows the PC ordination of the 16 atelid species based on shape plus logCS (form PC) variation. The results show a similar pattern of form variation to that observed for the endocranial shape data, with Alouatta and Ateles on opposite sides of PC1. However, given its large size, Cartelles displays a more marked differentiation in endocranial form space than in shape space, showing large differences from all the extant species. Similarly, Caipora is farther away from Brachyteles and Ateles in form than in shape space, due to its larger size. A similar pattern is observed in the CAC versus RSC1 space where, again, Cartelles displays a noticeably marked differentiation from the extant species (Figure 4b).
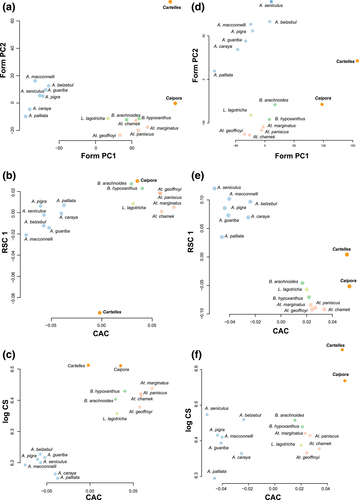
Finally, the plot of the CAC versus log Centroid size (logCS) shows the relationship between allometric shape variation and size among the atelid species. This reveals that for both Caipora and Cartelles, endocranial shape departs from the expected shape given their endocast size (Figure 4c). Importantly, this indicates that the unique endocranial shape of both fossils, but Cartelles in particular, cannot be explained merely by the effect of allometric scaling and size increase.
3.2 Cranial size and shape
Cranial shape variation in atelids is shown in Figures 2c,d and 3b and Figure S3b. Results of the PC ordination (Figure 2c,d)) reveal an overall pattern of variation that is largely similar to that observed for endocranial shape. However, whereas the Caipora cranium, similarly to its endocast, shows a strong resemblance to extant Brachyteles, the position of Cartelles in the cranial morphospace differs from that observed for its endocast. Particularly, while still occupying a region not represented by extant species, the Cartelles cranial shape shows a more intermediate morphology among atelids, slightly closer to atelins than to Alouatta. The minimum spanning tree links Cartelles with both Lagothrix and A. palliata, but the Procrustes distance is shorter between the fossil and the former (Figure S2b). The main difference between the cranial landmark set and the endocranial landmark set is the inclusion of landmarks on the facial skeleton in the former; it would appear that the addition of data from this part of the cranium is driving Cartelles farther toward the atelin side of the distribution along PC1.
In fact, the differences in cranial shape among the individuals along the first PC axis are mostly related to the relative size increase of the facial skeleton with respect to the neurocranium in the genus Alouatta. The relative position of the face (more anteriorly projected in Alouatta), together with the degree of flexion of the cranial base (less flexed in Alouatta; Figure 3b; Figure S3b), also contributes to the variation on PC1. Shape variation along PC2 mostly involves global changes in the facial and neurocranial skeleton within each genus (Figure 3b; Figure S3b).
Form PC, CAC versus RSC1, and CAC versus logCS (Figure 4d,f) show a similar picture, with Cartelles exhibiting a cranial morphology not as unique as its endocranial morphology. Nonetheless, given their large size, both fossil species stand out with respect to the extant species in the analyses including size as a variable. Noticeably, they resemble each other more in cranial morphology than in endocranial morphology (Figure 4).
4 DISCUSSION
The pattern of variation seen in the extant atelid species for cranial and endocranial shape, form, CAC and RSC is similar to the variation observed in previous studies on the cranium alone, showing a large difference between Alouatta and the other atelid genera (Aristide, dos Reis, et al., 2015; Fleagle et al., 2016; Halenar et al., 2017; Perez et al., 2011). Regarding the fossil species, we show that whereas Caipora bambuiorum is within the observed range of variation of extant Brachyteles, Cartelles coimbrafilhoi occupies a region of endocranial form and endocranial shape space that does not overlap with the range of variation seen in any of the extant taxa. Of the four genera, it is closest to Alouatta in endocranial shape space, but closest to Lagothrix in cranial shape space. This combination of associations with Alouatta and Lagothrix has been suggested by several previous studies (i.e., Halenar, 2011b, 2012; Halenar & Rosenberger, 2013). However, when form, CAC and RSC variation are explored, and size is taken into account, Cartelles appears much more different compared with the extant taxa, as well as with Caipora.
Differences between extant and fossil platyrrhine species have been observed in previous works. Halenar et al. (2017) explored cranial shape variation in extant and fossil platyrrhines and showed that several fossil specimens, including Cartelles, do not overlap with the distributions of extant atelid genera, but occupy a unique position in shape space, intermediate between Alouatta and the rest of the family. Similar results were found for Cartelles by Fleagle et al. (2016) using a comparative sample expanded to all primates. This suggests that the overall anatomy, and hence probably the niche occupied by Cartelles, have no equivalent among extant platyrrhine primates. Similar conclusions have been drawn based on analyses of Cartelles postcranial anatomy (i.e., Halenar, 2011b) and its relatively massive body size points to the same conclusion.
The similarities seen between Cartelles and Alouatta in endocranial shape (Krupp et al., 2012; this study) and hip morphology (Halenar & Rosenberger, 2013), together with forelimb anatomy (Halenar, 2011b; Hartwig & Cartelle, 1996; Jones, 2008) and dentition (Rosenberger et al., 2015) closer to that of atelins, suggest that this fossil species could be an example of sequential rather than simultaneous evolutionary changes in cranial and postcranial platyrrhine anatomies (Hartwig & Cartelle, 1996). If this is the case, what are the modalities and the direction of these changes? Halenar and Rosenberger (2013) suggested that Cartelles is a basal alouattine exhibiting the frugivorous dentition of ancestral atelids in combination with derived Alouatta-like traits in the cranium and the postcranium.
Caipora, on the other hand, is more closely comparable to extant forms, but the specific association of Caipora and Brachyteles was surprising. Upon its discovery, Caipora was suggested to be very similar to the extant spider monkey, Ateles, in cranial, dental, and postcranial morphology (Cartelle & Hartwig, 1996). The round globular braincase, bunodont dentition, and high intermembral index are phenetic resemblances shared most clearly with Ateles. The first clue that this relationship may not be so clear cut as originally assumed came when Rocatti et al. (2017) showed that Caipora is intermediate in mandible shape between Ateles and Brachyteles. The similarities seen between Caipora and Brachyteles in the results of this study, particularly in endocast shape, are intriguing given that the dentition observed in the fossil is undoubtedly similar to that of a frugivorous species, contrasting with the folivorous dentition of Brachyteles. The addition of facial landmarks, which capture more of this dental architecture, pulls the fossil closer to the range of variation of Ateles cranial morphology (Figure 2c,d).
These results speak to the question of which ecological factors may have driven the diversification of the members of the atelid clade. As discussed above, previous works have suggested—based on dental evidence—that the two fossils included here could have been mainly frugivorous. If we consider that: (a) Cartelles has been interpreted as the more primitive version of the pattern seen in extant Alouatta and Caipora could reflect the same for Brachyteles (Halenar & Rosenberger, 2013) and (b) it has long been proposed that Brachyteles and Alouatta evolved folivory independently (i.e., Rosenberger & Strier, 1989), it is possible that the fossils can be interpreted as the starting points of parallel dietary transitions to folivory. In further support of this suggested scenario, while dental adaptations to folivory are in place in the extant taxa, neither Alouatta nor Brachyteles have the digestive system adaptations to truly committed folivory as observed in colobine monkeys, for example. Accordingly, it has been hypothesized that both atelids evolved folivory from a frugivorous ancestor, perhaps via a seed-eating stage (Rosenberger et al., 2011). While changing dietary adaptations could have played some role in the evolutionary history of atelids, other factors, including the influence of the uniquely enlarged hyo-laryngeal apparatus in Alouatta, which may have already existed in Cartelles (Halenar, 2011b; Rosenberger et al., 2011, 2015), would need to be invoked to explain the highly divergent cranial anatomies exhibited in the two extant genera.
Alternatively, the differences and similarities in cranial and endocranial shape between the two fossil specimens and the extant species could be related to social behavior in the extinct taxa. Aristide et al. (2016) showed a relationship between group size (as a measure of social interaction complexity; Dunbar, 1998) and endocranial shape variation (as an indirect measure of brain shape). However, “social complexity” is a difficult concept to quantify, and group size may represent only a crude proxy for this variable. Because of this, recent comparative analyses in which social or ecological variables are used to explain brain morphological variation must be considered cautiously and need to be improved using various approaches (Healy & Rowe, 2007). Nevertheless, some fundamental relationships have emerged. For example, absolute brain size correlates with performance of non-human primates on a test of mental flexibility—the Transfer Index (transfer-of-learning)—and appears to be the most practical measure for distinguishing cognitive skills in a broad primatological context (Gibson, Rumbaugh, & Beran, 2001; Rumbaugh, Savage-Rumbaugh, & Washburn, 1996). In contrast, measures of relative brain size correlate with quantifications of dietary ecology (DeCasien, Williams, & Higham, 2017) and may therefore reflect a combination of responses to metabolic needs and cognitive strategies (Gibson et al., 2001). Moreover, Aristide et al. (2016) also showed that, as the brains (and endocast) are not uniform structures, endocranial shape (i.e., the relative position and size of selected individual brain structures) responds to changes in group size in more complex ways than overall brain size, and that the changes in the neocortex region have a stronger relationship with social group size (Aristide et al., 2016).
Both Cartelles and Caipora display endocasts suggesting intermediate development of the neocortex region compared to extant taxa. However, whereas Caipora shares aspects of endocranial shape with Brachyteles species, Cartelles has a unique morphology. While this may suggest that Caipora had large, complex social groups similar to Brachyteles, whether these morphological differences can be directly used to infer specifics regarding social behavior is currently unknown. This is particularly true in light of recent work in hominids showing effects of cranial anatomy on endocranial variation that were unrelated to actual brain morphology (i.e., Zollikofer, Bienvenu, & Ponce de León, 2017). However, when considering fossils, endocranial morphology is the only available proxy for brain shape and, if considered with caution, this parameter can still serve as a valuable source of data for the study of genetic/developmental determinants of brain structure, or for the study of the ethological relevance of brain structure (Ge et al., 2016).
The results of this study show the importance of considering both extant and fossil species when exploring diversification in complex traits, such as endocranial and cranial shape variation. Several recent studies analyzed cranial and mandibular shape variation in extant and fossil species of primates and other mammalian clades (e.g., Álvarez, Perez, & Verzi, 2011; Fleagle et al., 2016; Gunz et al., 2009; Püschel, Gladman, Bobe, & Sellers, 2017; Rocatti et al., 2017), and they also show the importance of fossil variation for understanding clade evolution. In particular, the joint use of data from fossil and extant species allows us to consider variation from periods predating or concomitant with events of mass extinction. Our results suggest that within the atelid clade, the extinction of Caipora bambuiorum and Cartelles coimbrafilhoi led to a loss of significant biological variation that could not have been imagined without the discovery of these fossils. To what extent the loss of this variation—without knowing these fossils—would significantly alter our understanding of the evolution of the atelid clade in particular, and of platyrrhines more generally, should be discussed more in depth in future studies. We suggest that questions about the ecological and evolutionary factors driving the diversification of the Atelidae cannot be fully answered through the sole study of extant species.
ACKNOWLEDGMENTS
The authors thank the Hermes Pardini Institute for allowing the use of their CT-scan facilities. Luciano Santos and Rodrigo Oliveira were instrumental in handling the fossil specimens. Two anonymous reviewers contributed greatly to improving the manuscript.




