Dental microwear of living Hadza foragers
Funding information: The Leakey Foundation; University of Arkansas; University of Nevada, Las Vegas
Abstract
Objectives
Studies of dental microwear of bioarchaeological assemblages and extant mammals from museum collections show that surface texture can provide a valuable proxy for reconstructing diets of past peoples and extinct species. However, no study to date has focused on occlusal surface microwear textures of living hunter-gatherers. Here we present the first such study of the Hadza foragers of Tanzania.
Methods
We took high-resolution dental impressions of occlusal surfaces for a total of 43 molds representing 25 men and women, 1–3 samples each, at different times during the rainy and dry seasons. Dental replicas were prepared and scanned by confocal profilometry and standard microwear texture parameters were calculated. Central tendencies and dispersions of variable scores were compared by season and by sex.
Results
We found no differences between sexes or seasons in texture attribute central tendency, but some for dispersion. Females had notably low microwear texture dispersion in the dry season while males had higher dispersion in some attributes, particularly in the dry season. These differences seem to be driven primarily by low variance among females in the dry season.
Conclusions
This study demonstrates microwear texture data can be generated for living foragers. Given caveats of small samples available and consideration of foraging groups in transition, this study hints at variation in microwear texture dispersion between sexes and seasons for the Hadza, suggesting that such analyses might be of value for assessing hunter-gatherer diet.
1 INTRODUCTION
Dental microwear texture analysis has proven to be a valuable tool for reconstructing the diets of bioarchaeological assemblages and fossil mammals (Calandra & Merceron, 2016; DeSantis, 2016; Schmidt, Beach, McKinley, & Eng, 2016; Ungar, 2015). With few exceptions, though, interpretation of texture patterns has relied on associations between diets reported in the literature for species or populations and specimens archived in museum collections. Museum specimens typically include limited provenience metadata, and thus permit little more than general associations between microwear pattern and inferred diet or subsistence practice.
Nevertheless, such studies show that microwear patterns vary with reported diets in predictable ways. Most bioarchaeological applications have used traditional measurement protocols, wherein scratches and pits are counted and measured directly from SEM photomicrographs. Cereal grains, for example, seem to leave distinctive patterns of microscopic feature size and shape that separate early agriculturalists from their hunter-gatherer forebears (e.g., Bullington, 1991; Mahoney, 2006; Pastor, 1993; Teaford, 1991). These and other studies suggest that microwear differences reflect food fracture properties (e.g., hard versus soft or tough foods) and food preparation technology (e.g., use of grinding stones) (Harmon & Rose, 1988; Molleson, Jones, & Jones, 1993; Teaford, Larsen, Pastor, & Noble, 2001).
Many microwear researchers today, especially those focusing on mammals, prefer texture analysis to feature-based study (Calandra & Merceron, 2016; DeSantis, 2016; Ungar, 2015). Microwear texture analysis characterizes surfaces represented by point clouds generated using confocal profilometry. This approach minimizes interobserver measurement error and allows 3D characterization and comparison of whole surface textures. Both scale-sensitive fractal geometry (Scott et al., 2006) and International Organization of Standardization (ISO 25178-2) parameters (Calandra, Schulz, Pinnow, Krohn, & Kaiser, 2012) are used to identify differences in surface texture between groups that vary in diet.
A few bioarchaeological studies have employed microwear texture analysis (El Zaatari, 2010; El Zaatari & Hublin, 2014; Krueger, 2014; Schmidt et al., 2016). El Zaatari focused on recent and Upper Palaeolithic foragers and found that groups consuming more plant matter and less meat evince higher molar microwear texture complexity (El Zaatari, 2010; El Zaatari & Hublin, 2014). Schmidt et al. (2016) likewise reported that pastoralists have lower molar microwear texture complexity, anisotropy, and textural fill volume (a measure of feature size) than do agriculturalists. Krueger extended bioarchaeological microwear texture study to incisors and reported that peoples known to use their anterior teeth in paramasticatory behaviors (e.g., arctic groups) differ in incisor microwear texture patterns from those who use their front teeth for ingestion only (Krueger, 2014; Krueger & Ungar, 2009).
Microwear texture analyses of recent humans have, to date, been limited to museum collections derived from bioarchaeological contexts with little metadata on diet or tooth use. Indeed, the only published microwear study of living human foragers that we know of involved a feature-based analysis of buccal surfaces by Romero, Ramirez-Rozzi, De Juan, and Perez-Perez (2013). That study compared microwear on the teeth of living Baka Pygmies to samples from skeletal collections, both hunter-gatherers and agriculturalists, and found differences in buccal striation density, presumably a result of stone-ground foods consumed by farmers but not foragers. This suggests the potential of microwear analysis for living peoples.
Here, we report on the first microwear texture analysis of occlusal surfaces of living foragers, the Hadza of Tanzania. This study seeks to determine: (a) whether occlusal surface microwear textures can be reliably reproduced in dental impressions taken from hunter-gatherers in the bush and, if so, (b) whether microwear texture patterns vary between males and females or between samples obtained at different times of year in a manner expected given reported differences in diet by sex and season.
2 MATERIALS AND METHODS
2.1 Human research subjects approval
Human Research Subjects approval was obtained from both the University of Nevada, Las Vegas Institutional Review Board in the Office of Research Integrity and the University of Arkansas Office of Research Compliance. Informed consent was obtained orally from all participants, as the Hadza are a largely nonliterate population. The IRB offices at both universities and the necessary Tanzanian government agencies approved the consent procedure. All data were collected with permission from the Tanzanian Commission for Science and Technology and the Tanzanian National Institute for Medical Research.
2.2 Study population
The Hadza hunter-gatherers occupy a 4000 km2 area around Lake Eyasi in northern Tanzania. They have a strong sexual division of labor wherein women have historically focused all food collection on plants (e.g., baobab fruit, berries, greens, and several species of tubers), while men target honey and larvae of both stingless and stinging bees and hunt the familiar fauna of East African ecosystems using bows and arrows (Crittenden, 2016). The total population of Hadzane speakers (the language of the Hadza) was reported to be about 1,000 individuals in 2000 (Blurton Jones, 2016). At the time of data collection only around 150 individuals were still practicing a principally hunting and gathering lifestyle, with a significant percentage of their diet derived from wild foods, while the remaining approximately 850 individuals resided close to villages and practiced a mixed subsistence economy with variable contributions of wild and domesticated species. Even those Hadza currently residing in remote bush camps consume some domesticated cultigens part of the year (Crittenden et al., 2017; Gibbons, 2018; Pollom, 2018), which they obtain from trade, purchase, or donation from researchers (Marlowe, 2002), ethnotour companies, missionary groups, and NGOs (Yatsuka, 2015).
2.3 Specimen sampling
Data were collected in two bush camps wherein residents consumed a predominately wild-food diet at the time of data collection. These camps are located just a few kilometers apart (Figure 1), but a considerable distance from any village (location of bush camps: S 03°51.426′ E 034°58.834′). Data were also collected in a third intermediate mixed-subsistence camp where participants consumed a diet composed of both wild and domesticated foods (Figure 1). This camp was located closer to a village and, consequently, residents had more frequent interaction with neighboring pastoral populations and visiting missionaries who routinely donated food (location of intermediate camp closer to a village: S 03°50.691′ E 035°09.205′).
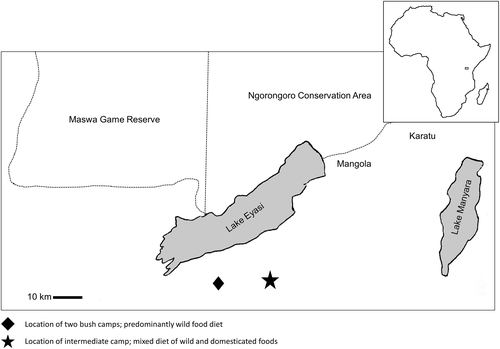
Our microwear data collection model called initially for us to sample an equal number of men and women, three times each, during the wet and dry seasons (samples to be taken at least 3 days apart). Field logistics made this sampling protocol impossible, as the Hadza are seminomadic hunter-gatherers. Because they moved frequently, not all of the same individuals were available in both seasons given variation in residence patterns. Also, because day-long foraging excursions are important for food acquisition in bush camps, some individuals were available only for one or two impressions per season. More importantly, some chose not to participate, and others were excluded from doing so given caries, broken teeth, or tooth sensitivity that would likely have caused discomfort during the molding process (Crittenden et al., 2017).
In the end, we were able to obtain 43 useable impressions that preserved “clean” microwear surfaces. These included 21 samples (n = 17 female, n = 4 male) from the wet season (January, 2015), and 22 samples (n = 10 female, n = 12 male) from the dry season (June, 2015). Useable specimens represented occlusal surfaces of 12 individuals during the wet season and 13 individuals during the dry season, each sampled 1–3 times. The breakdown by site was n = 5 for bush camp one, n = 22 for bush camp two, and n = 16 for intermediate camp three. Raw data for all samples separated by de-identified individual are presented as online supporting information.
2.4 Dental replication protocol
Microwear surface molding was challenging given the field conditions under which dental impressions were obtained. Aside from the lack of running water, electricity, and sterile laboratory setting, the Hadza were unfamiliar with dental procedures and averse to drawn out cleaning and molding, thus necessitating a rapid procedure. Furthermore, their teeth were often coated with a thick organic film and wax from chewing honeycomb, which was further exacerbated by lack of frequent dental cleaning and care.1 Dental biofilms were difficult to remove, and many of the impressions taken were not useable. Indeed, much of the current project focused on establishing and validating a technique capable of reproducing microwear surfaces for this community.
The dental impression protocol was developed in the laboratory prior to fieldwork with the goal of removing surface film without creating new microwear in the process. All participants were given one cup of popcorn to masticate, as this has been shown to remove organic film (Mark Teaford, pers comm). The popcorn was produced commercially and kept sealed in bags until consumption to minimize exposure to abrasive grit. Participants then rinsed with water, brushed their teeth with an unflavored prepasted toothbrush for 2 min, and rinsed again. Occlusal surfaces were then cleaned with an oral irrigation device (WP-300, Water-Pik, Inc), and teeth were dried with cotton balls and a portable electric air compressor (powered by a portable generator) fitted with a hose and intraoral nozzle for 2 min. Cotton balls were placed under the tongue to absorb saliva and keep the teeth dry.
Dental impressions were taken using polyvinylsiloxane (PVS) impression material (President microsystem Regular Body, Coltene-Whaledent) on the selected teeth (M1–2), with a tongue depressor placed on top of the PVS to keep the tongue away while the material set.
High-resolution replicas of specimens were later produced from these molds using Epotek 301 epoxy resin and hardener (Epoxy Technologies, Billerica, MA). All replicas were then screened for unobscured microwear using confocal profilometry (see below). Only surfaces preserving microwear fabrics at the micron scale were included in the analysis.
2.5 Specimen analysis protocol
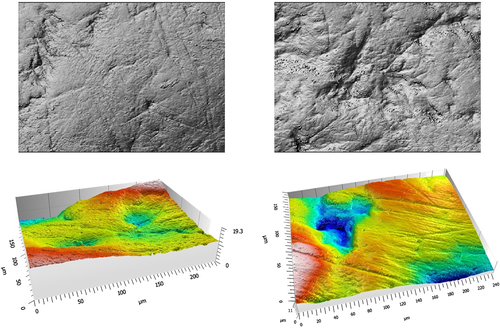
A Sensofar Plμ Neox confocal profiler (100× objective) was used to generate a digital elevation map of the “Phase II” facet 9 of each specimen (Krueger, Scott, & Ungar, 2008; Teaford & Walker, 1984). A single stitched point cloud of 242 μm × 181 μm with a lateral spacing of 0.17 μm was collected for each analyzed surface following recent protocol (Ungar et al., 2017). Sample surfaces are depicted in Figure 2.
Raw point clouds were processed using MountainsMap 6 (DigitalSurf, Besançon, France). Surfaces were leveled, and data representing dust or other adherent particles were deleted manually prior to analysis. A form removal algorithm was applied, and data spikes were removed by thresholding out the upper and lower 0.1% of data and soft-filtering (Spline filter according to ISO 16610-62, 2.5 μm cutoff) following Arman et al. (2016). Missing data points were filled using nearest a neighbor algorithm, as was required for generation of some of the microwear texture variables.
Each processed scan was analyzed using Toothfrax and SFrax (Surfract Corp, Norwich, VT) scale-sensitive fractal analysis (SSFA) software. Four surface texture variables were considered: area-scale fractal complexity (Asfc), scale of maximum complexity (Smc), length-scale anisotropy of relief (epLsar), and textural fill volume (Tfv). These together provide a texture snapshot that distinguishes surfaces with differing microwear feature sizes, shapes, and orientations and, in combination, provide a robust surface characterization appropriate for assessing differences in microwear pattern (Scott et al., 2006).
In addition, ISO 25178-2 parameters were calculated in MountainsMap 6 (Digital Surf Corp, Besançon, France). ISO parameters used here include skewness (Ssk), maximum peak height (Sp), maximum height (Sz), extreme peak height (Sxp), root mean square gradient (Sdq), developed interfacial area ratio (Sdr), pit void volume (Vvv), five-point pit height (S5v), mean dale area (Sda), and mean dale volume (Sdv). Descriptions of these measures and their mathematical underpinnings can be found in the surface metrology literature (e.g., Ţălu et al., 2014).
2.6 Dietary data
The research team conducted basic dietary interviews to determine general dietary patterns at the time of tooth molding. We asked participants to list the foods that they had eaten in the past 24 hr, the foods that they had eaten in the past week, and whether or not the weekly summary was an accurate reflection of their general diet over the preceding month.
2.7 Statistical procedures
Statistical procedures were designed to assess differences between sexes and seasons, both in central tendency and in dispersion. Small sample sizes prevented us from using multiple samples of individuals as replicates in a nested model comparing sex and season. It can be argued, however, that replicas of given individuals taken several days apart might be treated as separate samples in analyses. The replicas arguably represent different feeding bouts within a variable diet that might be expected to yield relatively rapid turnover in dental microwear (Teaford & Glander, 1991; Teaford & Oyen, 1988)—the so-called “last supper phenomenon” (Grine, 1986). While considering feeding bouts as separate data points (rather than individuals) is not ideal, the resulting increase in statistical power allows us to begin to assess possible season and sex differences. Raw data are provided by individual in the online supplement for comparison.
All statistical procedures were performed using Systat 12 (Systat Software, Inc., San Jose, CA). Variation in central tendency between wet and dry seasons and between males and females were assessed using a two-factor multivariate analysis of variance (MANOVA) model, with season and sex as the factors, and Ssk, Sp, Sz, Vvv, Sxp, Sdq, Sdr, S5v, Sda, Sdv, Asfc, Smc epLsar, and Tfv as the variables. Data were rank transformed to mitigate violation of assumptions inherent to parametric statistical tests (Conover & Iman, 1981), and Wilks' λ was used to assess variation by season, sex, and the interaction between these two factors.
Variation in dispersion was assessed using equality of two variances tests in Systat. Tests were conducted on raw data for each variable to compare seasons for the overall sample, males, and females separately. Tests were also conducted on each variable to compare sexes for the overall sample, wet season, and dry season separately. Experiment-wise error rates were not used because hypotheses and expectations for the different sexes and seasons are independent of one another and because adjustments of statistical significance would unnecessarily increase the likelihood of type II error (Perneger, 1998).
Finally, given the number of variables considered and the fact that some are likely to be correlated, we ran a principal component analysis to reduce the number of dimensions considered. We then analyzed PC1 and PC2 (again, rank transformed data) following the same procedures used on individual texture attributes.
3 RESULTS
Based on retrospective reporting of foods included in the diet the week prior to data collection, general dietary composition was estimated as follows. During the wet season, participants in all camps reported consumption of honey, larvae, baobab fruit, tubers, mammalian game meat, avian meat, small amounts of maize (donated from missionaries and tour guide operators), and pombe (a local alcoholic drink). During the dry season, participants in all camps reported consumption of honey, larvae, baobab fruit, tubers, berries, avian meat, small amounts of maize or barley, and pombe. In the camp located closer to a village, participants also reported the consumption of nutrition bars donated from missionaries. Notably absent in the dry season dietary recall is mammalian game meat, which is historically when meat consumption is at its highest (Berbesque & Marlowe, 2009).
Summary statistics for samples separated by season and sex are presented in Table 1. Results from the two-factor MANOVA test show no significant variation in the model (Table 2). While we found no difference in microwear texture central tendencies between the sexes, between the seasons, or as an interaction effect between the two factors, we did find differences between sex and season in dispersion of microwear texture variables associated with feature size and shape (Table 3, Figure 3). The pooled sex sample shows differences between wet and dry season in Sda, and Sdv. These texture attributes show less dispersion in the wet season than in the dry season. The male sample also differed between seasons in Sdv, again with values lower in the wet season than the dry season (with the caveat that the male sample is much smaller for the wet season than for the dry). The female sample differed between seasons in Sdv, Sdr, Sdq, and Asfc. In these cases, however, wet season dispersion is greater than dry season dispersion.
| Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 27) | Wet season (n = 17) | Dry season (n = 10) | |||||||
| Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | |
| Ssk | −0.374 | −0.502 | 0.572 | −0.276 | −0.477 | 0.649 | −0.572 | −0.544 | 0.441 |
| Sp | 3.450 | 3.870 | 1.911 | 3.650 | 4.476 | 2.038 | 2.605 | 2.840 | 1.137 |
| Sz | 7.980 | 8.804 | 3.793 | 9.000 | 10.080 | 4.061 | 6.360 | 6.634 | 1.981 |
| Vvv | 0.147 | 0.173 | 0.107 | 0.173 | 0.195 | 0.120 | 0.128 | 0.137 | 0.074 |
| Sxp | 2.270 | 2.803 | 1.635 | 3.010 | 3.151 | 1.787 | 1.995 | 2.211 | 1.194 |
| Sdq | 0.198 | 0.219 | 0.060 | 0.211 | 0.232 | 0.071 | 0.196 | 0.196 | 0.026 |
| Sdr | 1.820 | 2.178 | 1.019 | 2.050 | 2.411 | 1.198 | 1.795 | 1.783 | 0.419 |
| S5v | 2.380 | 2.740 | 1.369 | 3.060 | 2.937 | 1.521 | 2.305 | 2.404 | 1.050 |
| Sda | 955.000 | 1,023.778 | 435.815 | 1,159 | 1,118 | 480.788 | 865.500 | 863.600 | 304.101 |
| Sdv | 26.600 | 40.453 | 39.148 | 31.600 | 50.007 | 46.263 | 21.150 | 24.210 | 12.530 |
| Asfc | 2.187 | 2.591 | 1.174 | 2.568 | 2.870 | 1.386 | 2.153 | 2.118 | 0.415 |
| Smc | 0.220 | 0.314 | 0.207 | 0.220 | 0.330 | 0.228 | 0.220 | 0.287 | 0.174 |
| epLsar | 0.004 | 0.003 | 0.002 | 0.004 | 0.004 | 0.002 | 0.001 | 0.002 | 0.002 |
| Tfv | 49,636.860 | 49,772.290 | 6,529.746 | 51,092.010 | 49,489.720 | 6,832.245 | 46,977.330 | 50,252.660 | 6,307.305 |
| Male | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 16) | Wet season (n = 4) | Dry season (n = 12) | |||||||
| Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | |
| Ssk | −0.500 | −0.508 | 0.372 | −0.462 | −0.441 | 0.236 | −0.500 | −0.531 | 0.414 |
| Sp | 3.225 | 3.713 | 3.039 | 3.120 | 3.793 | 2.550 | 3.455 | 3.687 | 3.289 |
| Sz | 8.430 | 8.541 | 4.980 | 6.535 | 8.367 | 5.233 | 9.015 | 8.599 | 5.133 |
| Vvv | 0.163 | 0.156 | 0.085 | 0.122 | 0.158 | 0.098 | 0.177 | 0.155 | 0.085 |
| Sxp | 2.600 | 2.536 | 1.370 | 1.995 | 2.475 | 1.484 | 2.835 | 2.557 | 1.400 |
| Sdq | 0.202 | 0.230 | 0.077 | 0.224 | 0.231 | 0.095 | 0.202 | 0.229 | 0.075 |
| Sdr | 1.830 | 2.515 | 1.478 | 2.370 | 2.577 | 1.778 | 1.830 | 2.494 | 1.455 |
| S5v | 3.330 | 2.944 | 1.511 | 2.700 | 2.712 | 1.596 | 3.330 | 3.021 | 1.547 |
| Sda | 820.00 | 1,065.563 | 849.404 | 825.500 | 837.000 | 314.283 | 686.500 | 1,141.75 | 965.183 |
| Sdv | 16.700 | 78.492 | 146.768 | 22.100 | 24.407 | 17.847 | 16.700 | 96.520 | 166.939 |
| Asfc | 2.267 | 3.167 | 1.823 | 3.180 | 3.443 | 2.304 | 2.267 | 3.075 | 1.745 |
| Smc | 0.220 | 0.337 | 0.259 | 0.172 | 0.241 | 0.176 | 0.220 | 0.369 | 0.280 |
| epLsar | 0.002 | 0.003 | 0.002 | 0.003 | 0.003 | 0.002 | 0.002 | 0.003 | 0.002 |
| Tfv | 51,855.785 | 53,951.017 | 14,054.870 | 51,111.180 | 53,501.930 | 14,181.160 | 51,855.785 | 54,100.714 | 14,643.333 |
| Male + female | ||||||
|---|---|---|---|---|---|---|
| Wet season (n = 12) | Dry season (n = 22) | |||||
| Median | Mean | SD | Median | Mean | SD | |
| Ssk | −0.282 | −0.470 | 0.588 | −0.500 | −0.537 | 0.416 |
| Sp | 3.620 | 4.346 | 2.092 | 3.085 | 3.302 | 2.531 |
| Sz | 8.810 | 9.754 | 4.216 | 6.710 | 7.706 | 4.060 |
| Vvv | 0.171 | 0.188 | 0.115 | 0.134 | 0.147 | 0.079 |
| Sxp | 2.680 | 3.022 | 1.720 | 2.045 | 2.400 | 1.292 |
| Sdq | 0.211 | 0.232 | 0.073 | 0.196 | 0.214 | 0.059 |
| Sdr | 2.050 | 2.442 | 1.275 | 1.795 | 2.171 | 1.147 |
| S5v | 3.060 | 2.894 | 1.497 | 2.345 | 2.741 | 1.351 |
| Sda | 1,002 | 1,064.476 | 461.005 | 831.000 | 1,015.318 | 740.067 |
| Sdv | 31.600 | 45.131 | 43.198 | 19.505 | 63.652 | 126.583 |
| Asfc | 2.568 | 2.979 | 1.545 | 2.175 | 2.640 | 1.381 |
| Smc | 0.220 | 0.313 | 0.218 | 0.220 | 0.331 | 0.236 |
| epLsar | 0.004 | 0.004 | 0.002 | 0.002 | 0.003 | 0.002 |
| Tfv | 51,092.010 | 50,253.950 | 8,373.515 | 48,942.906 | 52,351.600 | 11,541.862 |
| Wilks' λ | F | df | p | |
|---|---|---|---|---|
| Season | 0.739 | 0.656 | 14, 26 | .794 |
| Sex | 0.716 | 0.738 | 14, 26 | .720 |
| Interaction | 0.664 | 0.941 | 14, 26 | .532 |
| Season (comparison of wet versus dry) | ||||||
|---|---|---|---|---|---|---|
| Overall (df = 21, 20) | Female (df = 9, 16) | Male (df = 11, 3) | ||||
| F | p | F | p | F | p | |
| Ssk | 0.501 | .124 | 0.461 | .240 | 3.084 | .384 |
| Sp | 1.464 | .398 | 0.311 | .081 | 1.663 | .745 |
| Sz | 0.927 | .863 | 0.238 | .034 | 0.962 | .827 |
| Vvv | 0.470 | .093 | 0.382 | .146 | 0.745 | .621 |
| Sxp | 0.564 | .201 | 0.446 | .221 | 0.890 | .763 |
| Sdq | 0.656 | .345 | 0.134 | .004 | 0.624 | .490 |
| Sdr | 0.809 | .632 | 0.122 | .003 | 0.670 | .541 |
| S5v | 0.814 | .643 | 0.476 | .260 | 0.939 | .807 |
| Sda | 2.577 | .039 | 0.400 | .166 | 9.431 | .090 |
| Sdv | 8.587 | .000 | 0.073 | .000 | 87.494 | .004 |
| Asfc | 0.799 | .613 | 0.090 | .001 | 0.574 | .432 |
| Smc | 1.175 | .722 | 0.584 | .416 | 2.531 | .482 |
| epLsar | 1.292 | .570 | 1.351 | .574 | 1.286 | .939 |
| Tfv | 1.900 | .157 | 0.852 | .835 | 1.066 | .910 |
| Sex (comparison of males versus females) | ||||||
|---|---|---|---|---|---|---|
| Overall (df = 26, 15) | Dry (df = 9, 11) | Wet (df 16, 3) | ||||
| F | p | F | p | F | p | |
| Ssk | 2.365 | .085 | 1.133 | .831 | 7.573 | .121 |
| Sp | 0.396 | .037 | 0.120 | .004 | 0.639 | .473 |
| Sz | 0.580 | .216 | 0.149 | .008 | 0.602 | .431 |
| Vvv | 1.607 | .339 | 0.767 | .701 | 1.494 | .834 |
| Sxp | 1.423 | .481 | 0.728 | .644 | 1.451 | .857 |
| Sdq | 0.614 | .267 | 0.102 | .004 | 0.558 | .378 |
| Sdr | 0.475 | .093 | 0.083 | .001 | 0.454 | .255 |
| S5v | 0.820 | .638 | 0.460 | .254 | 0.908 | .754 |
| Sda | 0.263 | .003 | 0.099 | .002 | 2.340 | .528 |
| Sdv | 0.071 | .000 | 0.006 | .000 | 6.719 | .142 |
| Asfc | 0.415 | .048 | 0.057 | .000 | 0.362 | .152 |
| Smc | 0.642 | .312 | 0.387 | .165 | 1.679 | .746 |
| epLsar | 1.087 | .890 | 1.003 | .979 | 0.955 | .797 |
| Tfv | 0.216 | .001 | 0.017 | .000 | 0.232 | .042 |
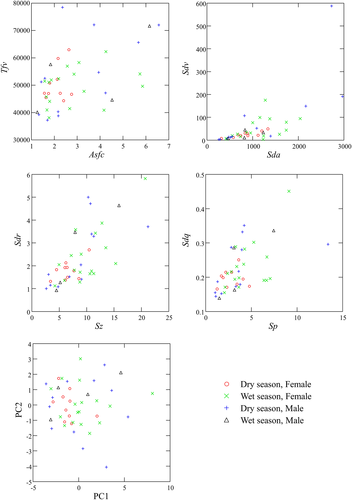
There are other hints that microwear dispersion pattern differs between males and females. Indeed, for the pooled season sample, the sexes differ in Sp, Sda, Sdv, Asfc, and Tfv. In all cases, females show less dispersion than their male counterparts. There are significant differences in the dry season between sexes for even more variables, including Sp, Sda, Sdv, Asfc, and Tfv, but also Sz, Sdq, and Sdr. In all cases, again, females have less dispersion in microwear texture values than do males. There is only one sex difference for the wet season, with females showing more dispersion than males for Tfv, but we caution against overinterpretation of the wet season result given the much smaller male sample than female one. Indeed, all results should be interpreted in light of assumptions of the equal variance test and uneven sample sizes. It is possible, for example, that the higher variance in females for wet season compared with dry season results reflects, in part, larger samples. On the other hand, for the five pooled-season tests showing variation in dispersion between sexes, males have consistently higher variances despite smaller samples.
Similar results were found using the PCA (Table 4). Sp, Sz, Vvv, Sxp, Sdq, Sdr, S5v, and Asfc all presented high loading values for PC1 and Sda and Sdv presented moderate loadings for PC2. Nearly 47% of the variance was explained by PC1, and 15% by PC2. MANOVA results showed no significant variation in central tendency between the groups by sex or season, and no interaction between the two factors. Differences in dispersion between sexes and seasons seem to be driven largely by low variance for females in the dry season. Indeed, dry-season males had more dispersion in both PC1 and PC2 values than did dry-season females; and wet-season females had higher dispersion along PC1 than did dry-season females. Finally, males generally had more dispersion in PC2 than females. There were no other significant differences between sexes or seasons in dispersion, with the caveat of a small sample of wet-season males.
| A. Component loadings | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Ssk | −0.152 | 0.387 | 0.736 | 0.028 | 0.511 | −0.057 | 0.022 | 0.012 | 0.016 | 0.135 | −0.032 | −0.012 | 0.002 |
| Sp | 0.755 | 0.001 | 0.572 | 0.092 | 0.053 | −0.136 | −0.173 | 0.021 | −0.079 | −0.177 | 0.071 | 0.034 | 0.004 |
| Sz | 0.923 | −0.107 | 0.200 | 0.091 | −0.168 | −0.029 | −0.140 | 0.085 | −0.078 | −0.128 | −0.084 | −0.060 | −0.005 |
| Vvv | 0.910 | −0.169 | −0.005 | 0.236 | −0.045 | −0.151 | 0.018 | −0.230 | 0.060 | 0.045 | −0.002 | 0.015 | 0.040 |
| Sxp | 0.923 | −0.140 | 0.006 | 0.223 | 0.007 | −0.149 | 0.034 | −0.216 | 0.057 | 0.058 | −0.005 | 0.016 | −0.042 |
| Sdq | 0.924 | 0.246 | −0.160 | −0.004 | 0.098 | 0.098 | 0.172 | 0.029 | 0.047 | 0.023 | 0.048 | −0.075 | 0.012 |
| Sdr | 0.905 | 0.285 | −0.174 | −0.069 | 0.139 | 0.106 | 0.157 | 0.051 | 0.058 | −0.010 | 0.028 | −0.019 | −0.013 |
| S5v | 0.863 | −0.163 | −0.121 | 0.253 | −0.103 | −0.016 | −0.121 | 0.232 | −0.143 | 0.222 | 0.008 | 0.029 | 0.001 |
| Sda | 0.324 | −0.672 | 0.402 | −0.402 | −0.198 | 0.052 | −0.035 | 0.102 | 0.245 | 0.052 | 0.005 | 0.003 | 0.001 |
| Sdv | 0.339 | −0.605 | 0.107 | −0.561 | 0.137 | 0.236 | 0.242 | −0.127 | −0.208 | 0.023 | −0.001 | 0.000 | 0.000 |
| Asfc | 0.794 | 0.419 | −0.180 | −0.193 | 0.141 | 0.174 | 0.207 | 0.104 | 0.060 | −0.092 | −0.047 | 0.076 | 0.004 |
| Smc | −0.159 | −0.419 | 0.071 | 0.625 | 0.166 | 0.606 | −0.070 | −0.015 | 0.042 | −0.037 | 0.001 | 0.003 | 0.000 |
| epLsar | −0.142 | 0.480 | 0.489 | 0.166 | −0.549 | 0.186 | 0.377 | −0.041 | −0.035 | 0.041 | 0.004 | 0.005 | −0.001 |
| Tfv | 0.333 | 0.564 | 0.005 | −0.381 | −0.139 | 0.329 | −0.526 | −0.132 | −0.001 | 0.062 | 0.002 | 0.001 | 0.000 |
| Variance explained by components | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Variance | 6.546 | 2.096 | 1.431 | 1.268 | 0.759 | 0.688 | 0.647 | 0.226 | 0.155 | 0.140 | 0.019 | 0.018 | 0.004 |
| % Total | 46.758 | 14.969 | 10.223 | 9.061 | 5.423 | 4.916 | 4.618 | 1.611 | 1.110 | 1.001 | 0.135 | 0.129 | 0.027 |
| B. Central tendency two-factor MANOVA results (rank-transformed PC1 and PC2) | ||||
|---|---|---|---|---|
| Wilks' λ | F | df | p | |
| Season | 0.969 | 0.610 | 2, 38 | .549 |
| Sex | 0.982 | 0.339 | 2, 38 | .715 |
| Interaction | 0.947 | 1.066 | 2, 38 | .354 |
| C. Two-sample dispersion test results | |||||
|---|---|---|---|---|---|
| Sample | Comparison | df | F | p | |
| PC1 | All | Season | 21, 20 | 0.867 | .747 |
| All | Sex | 26, 15 | 0.551 | .177 | |
| Female | Season | 9, 16 | 0.251 | .041 | |
| Male | Season | 11, 3 | 0.647 | .647 | |
| Dry | Sex | 9, 11 | 0.172 | .013 | |
| Wet | Sex | 16, 3 | 0.527 | .342 | |
| PC2 | All | Season | 21, 20 | 1.507 | .364 |
| All | Sex | 26, 15 | 0.390 | .034 | |
| Female | Season | 9, 16 | 0.550 | .365 | |
| Male | Season | 11, 3 | 2.495 | .490 | |
| Dry | Sex | 9, 11 | 0.228 | .035 | |
| Wet | Sex | 16, 3 | 0.527 | .866 | |
4 DISCUSSION
Analyses of extant mammals have demonstrated consistent and predictable associations between microwear texture pattern and diet (see Calandra & Merceron, 2016; DeSantis, 2016; Ungar, 2015). Those species that consume harder, more brittle foods tend to have more complex, pitted surfaces whereas those that eat softer, tougher foods on average tend to have more anisotropic, striated surfaces. These evidently relate to the notion that pits are more likely to form when opposing surfaces are pressed together (e.g., when crushing hard foods) whereas aligned scratches are expected when opposing surfaces slide past one another (e.g., when shearing tough items) (Hua, Brandt, Meullenet, Zhou, & Ungar, 2015).
Dental microwear texture analyses of recent human populations also show differences in central tendency that track diet. For example, El Zaatari's (2010) analyses of historic hunter-gatherer groups indicate significant variation in microwear texture central tendencies (Asfc, Smc, epLsar, and Tfv) by percentage of meat in the diet. In another study, Schmidt et al. (2016) found pastoralists to have significantly lower texture values for all the variables they considered (Asfc, epLsar, and Tfv) compared to agriculturalists. All signs to date suggest that microwear textures reflect differences in diet among humans with differing diets.
While previous work among the Hadza demonstrated sex differences in gross dental wear patterning (Berbesque et al., 2012) that may be associated with diet composition or tooth use, our results failed to show significant variation in microwear texture central tendencies by either sex or season, consistent with similar modal diets across seasons and between sexes. In contrast, the consistently lower dispersion of values for females in the dry season (compared both with males in the dry season and females in the wet season) does suggest that there may be differences between seasons and sexes in the breadth of foods consumed that yield different microwear patterns. Unfortunately, our dietary recall metadata were not precise enough to identify differences in diet breadth between individuals over the course of days reflected in the microwear.
The past 50 years has yielded comprehensive dietary data collected among bush living Hadza foragers (Crittenden, 2016; Marlowe, 2010; Marlowe & Berbesque, 2009; Murray, Schoeninger, Bunn, Pickering, & Marlett, 2001; Schoeninger, Bunn, Murray, & Marlett, 2001; Vincent, 1985). A general pattern of seasonal dietary diversity replicated with each published study suggests that honey and larvae are more available during the rainy season, game meat is more available during the dry season, and plant foods (baobab, berries, and tubers) are available in differing amounts year round with a tendency for berries to be consumed in greater quantities when tubers are less available and vice versa (Marlowe & Berbesque, 2009). Furthermore, sex differences in diet have been reported for bush dwelling Hadza consuming a largely wild-food diet (Berbesque & Marlowe, 2009; Berbesque, Marlowe, & Crittenden, 2011).
Despite this general pattern holding true for studies reporting on diet composition prior to 2010, our data may be consistent with a different story—one of a rapidly shifting Hadza diet. Data on oral health and diet collected during the same time period as the current study suggest that even bush-residing Hadza consume increasing amounts of domesticated cultigens with each passing year. Elsewhere we have demonstrated the effects of increasing domestication on teeth in comparisons of oral health between Hadza in bush camps and those in villages (Crittenden et al., 2017). Women living in villages consuming mostly maize exhibited more caries than those in the bush consuming a mostly wild food diet. Perhaps the microwear data presented here are indicative of a similar trend; in this case, a diet of increasing domesticated cultigens results in a less diverse diet—though microwear and oral health proxies operate at very different temporal scales and better dietary metadata are required to evaluate this hypothesis.
Dietary change is occurring at a rapid rate in Hadza land. Groups such as Carbon Tanzania (https://www.carbontanzania.com/) track land cover change from almost exclusively acacia-commiphora woodland (i.e., “bush”) to land used as, or converted to, agricultural land. As of 2015, much of the Gideru Hills region in Yaeda Valley, where the bush-dwelling Hadza reside, is now a combination of woodland and agriculture. Despite conservation efforts and land rights struggles, the Hadza now share their ancestral land with neighboring ethnic groups such as the Sukuma (subsistence farmers) and Datoga (pastoralists), even in the most remote locations once occupied only by the Hadza (Gibbons, 2018; Smith, 2015).
It is noteworthy that no participants reported the consumption of large mammalian game meat during the dry season (avian meat only). While data from the past several decades suggest that meat consumption in all parts of Hadza territory was greatest during the dry season (Berbesque & Marlowe, 2009; Blurton Jones, 2016; Marlowe, 2010), recent reports indicate that the diet of Hadza foragers is changing. Indeed, while the lack of differences in microwear texture central tendency might be an artifact of small sample sizes, uneven sampling, limitations of microwear to distinguish dietary differences, or the vagaries of capturing dietary moments in time for individuals with broad diets, it is also plausible that dietary homogenization related to subsistence change is, at least in part, responsible.
Our results are perhaps not unexpected, given the small sample sizes for different sexes and seasons and associated statistical limitations. While the absence of evidence is not necessarily evidence of absence, ongoing ecological and nutritional shifts suggest that these results may, in part, be rooted in dietary trends. The influx of farmed and processed foods, anthropogenic changes such as paved roads adjacent to Hadza land (Fyumagwa et al., 2013; Hopcraft et al., 2015), climate change (Mabulla, 2012), increased pressure from ecotour companies (Butovskaya, 2013), and the increased presence of missionaries and nongovernmental organizations (NGOs) (Yatsuka, 2015) all impact not only diet composition of Hadza foragers, but perhaps sex differences in consumption patterns as well. These dietary shifts may contribute to homogenization of diet between the sexes and the seasons and perhaps the differences in diet breadth reported here also.
This study presents the first occlusal surface microwear texture data, and joins Romero and colleagues' study (2013) as one of two investigations of microwear of living foragers. It suggests that occlusal surface microwear of living foragers in a savannah environment can be analyzed and potentially yield meaningful results. The methods outlined allow interested researchers to undertake microwear analysis on any living population—not a trivial task given the extremely resilient organic film that obscures occlusal surfaces.
ACKNOWLEDGMENTS
We are grateful to the Hadza for their research participation and hospitality and Mika Peterson and Audax Mabulla for logistical support in the field. We further extend our gratitude to Mark Teaford and Lucas Delezene for their very helpful suggestions on an earlier version of this article and to Colette Berbesque for her collegiality and willingness to offer insight gained during her previous work on Hadza dental wear patterns. We also acknowledge the Center for Academic Research and Training in Anthropogeny (CARTA) at the University California, San Diego for hosting the symposium on the evolution of human nutrition that inspired the project that led to this article. This study was funded by the LSB Leakey Foundation, The University of Nevada, Las Vegas (Faculty Opportunity Award Grant), and the University of Arkansas.




