Dental microwear texture analysis of Homo sapiens sapiens: Foragers, farmers, and pastoralists
Funding information British Academy/Leverhulme Trust; Faculty of Arts Doctoral Award, University of Auckland; Leakey Foundation, Marie Skłodowska-Curie Actions, Grant/Award Number: H2020-MSCA-IF-2016 No. 749188, AGAUR (Ref. 2017SGR1040) with URV (Ref. 2016PFR-URV-B2-17), and MINECO/FEDER (Ref. CGL2015-65387-C3-1-P); LUROP Mulcahy Fellowship, Loyola University; National Science Foundation, Grant/Award Numbers: BCS-0636066, BCS-0820805, BCS-0922930, BCS-1528698; Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant/Award Number: process 2013/00069-0; Wenner-Gren Foundation for Anthropological Research, Grant/Award Number: GR6698
Abstract
Objectives
The current study seeks to determine if a sample of foragers, farmers, and pastoralists are distinguishable based on their dental microwear texture signatures.
Materials and methods
The study included a sample of 719 individuals from 51 archeological sites (450 farmers, 192 foragers, 77 pastoralists). All were over age 12 and sexes were pooled. Using a Sensofar® white-light confocal profiler we collected dental microwear texture analysis (DMTA) data from a single first or second molar from each individual. We leveled and cleaned data clouds following standard procedures and analyzed the data with Sfrax® and Toothfrax® software. The DMTA variables were complexity and anisotropy. Statistics included ANOVA with partial eta squared and Hedges's g. We also performed a follow-up K-means cluster analysis.
Results
We found significant differences between foragers and farmers and pastoralists for complexity and anisotropy, with foragers having greater complexity than either the farmers or the pastoralists. The farmers and pastoralists had greater anisotropy than the foragers. The Old World foragers had significantly higher anisotropy values than New World foragers. Old and New World farmers did not differ. Among the Old World farmers, those dating from the Neolithic through the Late Bronze Age had higher complexity values than those from the Iron Age through the medieval period. The cluster analysis discerned foragers and farmers but also indicated similarity between hard food foragers and hard food farmers.
Discussion
Our findings reaffirm that DMTA is capable of distinguishing human diets. We found that foragers and farmers, in particular, differ in their microwear signatures across the globe. There are some exceptions, but nothing that would be unexpected given the range of human diets and food preparation techniques. This study indicates that in general DMTA is an efficacious means of paleodietary reconstruction in humans.
1 INTRODUCTION
The current study seeks to determine if a sample of foragers, farmers, and pastoralists can be distinguished by their microwear texture signatures. Dental microwear texture analysis (DMTA) is a method of dietary reconstruction based on interpreting micro-features as they appear on dental enamel. Since 2005, researchers have demonstrated its efficacy via comparative and experimental studies using extinct and extant organisms (e.g., Calandra, Schulz, Pinnow, Krohn, & Kaiser, 2012; Delezene, Teaford, & Ungar, 2016; DeSantis, Schubert, Scott, & Ungar, 2012; Estalrrich & Rosas, 2015; Purnell & Darras, 2016; Ragni, Teaford, & Ungar, 2017; Schulz, Calandra, & Kaiser, 2013; Scott et al., 2005; Scott et al., 2006; Scott, Teaford, & Ungar, 2012; Shearer et al., 2015; Teaford & Ungar, 2014; Teaford, Ungar, Taylor, Ross, & Vinyard, 2017; Ungar, Grine, & Teaford, 2008). Specifically, the DMTA derived from living primates has been especially integral in the contextualization of fossil primate and hominin diets (e.g., Delezene, Zolnierz, Teaford, Grine, & Ungar, 2013; El Zaatari, Grine, Ungar, & Hublin, 2016; Grine, Ungar, Teaford, & El Zaatari, 2013; Karriger, Schmidt, & Smith, 2016; Scott et al., 2005; Ungar, 2012; Ungar et al., 2008; Ungar, Krueger, Blumenschine, Njao, & Scott, 2012; Ungar & Scott, 2009; Ungar, Scott, & Steininger, 2016; Ungar & Sponheimer, 2011) and recent experimental work has allowed us to better understand the mechanics of microwear formation (e.g., Daegling, Hua, & Ungar, 2016; Hua, Brandt, Meullenet, Zhou, & Ungar, 2015; Xia et al., 2015, 2017).
Collectively, these studies support and clarify many comparative interpretations regarding extant and extinct species. Studies have applied DMTA to understand the dietary strategies of recent humans (e.g., El Zaatari, 2008, 2010; Schmidt, Beach, McKinley, & Eng, 2016; Schmidt, Chiu, Frazer, Barrett, & Mahoney, 2011; Schmidt & Remy, 2016; Scott, Halcrow, Standen, Arriaza, & Schmidt, 2016; Spengler, Da Gloria, & Schmidt, 2018; Willman, Schmidt, Remy, Shackleford, & Demeter, 2018); however, none have examined such data on hundreds of individuals on a global scale. Here we present data from just over 700 primarily Holocene humans that comprise a global sample (Figure 1) who represent three primary subsistence strategies: foraging, farming, and pastoralism. This study tests hypotheses designed to elucidate relationships between microwear texture and diet based upon current DMTA research. Success in this endeavor would provide bioarchaeologists with a robust toolkit for ascertaining changes in biocultural adaptive strategies. Since previous DMTA studies (e.g., Scott et al., 2005; Scott et al., 2006) have shown that two microwear variables—complexity and anisotropy—are particularly relevant in discerning diet, they are the focus here.
2 MECHANICS OF MICROWEAR
Masticatory microwear forms as the molar cusps interact with food. The chewing cycle begins as the mandible is elevated and slightly deviated laterally in order to initiate food breakdown. For the first several cycles, however, it is common for jaw movements to be relatively vertical, with the molars failing to come into contact. Called puncture-crushing, this phase reduces food to a point where it can be more finely chewed. Once the molars begin to interact, the power stroke begins. At first, in what is called Phase I, the upper and lower molar cusps glide past each other creating a shearing force as the mandible moves superiorly and medially. As the mandible nears centric occlusion Phase II begins. At this point, even though applied forces begin to drop, the mandibular molars tightly occlude with their maxillary counterparts, compressing food particles. Each cycle ends when the mandible moves just past centric occlusion (Hiiemae & Kay, 1972; Kay & Hiiemae, 1974; see also Ungar, 2015 for an overview). This process creates micro-abrasions and pits on occlusal surfaces that are usually no more than a few microns in diameter. Molar cusps exhibit these microscopic features on occlusal facets. Phase I facets tend to be dominated by scratch features created as the corresponding maxillary and mandibular cusps slide past one another (although at times Phase I facets may express little in the way of microwear). Phase II facets tend to have scratch and pit features because tight occlusion leads to both crushing and grinding (Krueger, Scott, Kay, & Ungar, 2008). It should be noted that, although pit and scratch are terms used periodically by DMTA analysts to provide visual descriptions of dental micro-features, they are not distinguished, measured, or counted using current DMTA methods.
DMTA is a particularly valuable indicator of diet because it provides a direct record of tooth–food–tooth interactions. Lucas et al. (2013) argued that only foods that are harder than enamel are capable of abrading it. However, recent experimental studies have determined that ingested materials need not be as hard as dental enamel to scratch it. The materials only need to be strong enough to break the protein bonds that hold together enamel crystallites (e.g., Xia et al., 2015, 2017). Foods such as meat, which have no hard particles in them, leave behind no microwear, while foods with fine particles (such as phytoliths) will generate microwear features (Hua et al., 2015; Krueger et al., 2018).
2.1 Grit
The addition of grit to a diet is a perennial concern. However, there are instances where grit may actually assist in microwear interpretation because it varies based on its source. For example, exogenous dust differs in size and concentration at different levels of a forest canopy (Ungar, Teaford, Glander, & Pastor, 1995), and controlled studies of ungulates show that grit size influences microwear patterning (Hoffman, Fraser, & Clementz, 2015). According to in vivo analysis of ungulate microwear, the presence of exogenous grit and material properties of foods consumed do influence microwear signatures; however, the material properties of foods were more influential on the overall pattern of microwear than exogenous grit (Merceron et al., 2016).
In humans, grit tends to vary based upon the types of tools used to mechanically process food, for example grinding stones made from sedimentary rocks tend to add more grit contamination than softer grinding items, like wooden pestles. Teaford and Lytle (1996) found in their experiment that stone-ground maize increased microwear formation when added to an otherwise modern diet. Grit is also found in wild foods that are not mechanically processed, including meat which can become contaminated (see El Zaatari, 2008, 2010). In fact, people who do not, or only slightly, mechanically process their food still exhibit rapid macrowear and microwear characterized by wide scratches (e.g., Schmidt, 2001, 2010).
When comparing grit from grinding stones to that from wild foods, the grinding stone microwear features are often smaller. Not surprisingly, those who use wooden tools tend to have the least amount of grit. For example, in North America wooden grinding tools were used more commonly during the agriculturally focused Mississippian period (Greenlee, 2009), which had a decrease in macrowear expression and microwear pitting (Schmidt, 2010). For this reason, when contextualizing human microwear it is important to account for food processing tools as well as floral and faunal remains associated with each population. This is because, unlike nearly all nonhuman microwear studies, microwear in modern Homo sapiens does not just reflect the food consumed; it also reflects the manners by which foods were processed. This is an unavoidable circumstance when studying humans and requires analysts to be aware that their dietary reconstructions actually mean food consumed + manner of processing. Fortunately, food processing is not random and its effects on teeth are becoming increasingly understood (e.g., El Zaatari, 2010; Hua et al., 2015).
3 CAVEATS
Regardless of the large body of work that highlights the efficacy of microwear analysis, critics have questioned its reliability due to its short-lived, “ephemeral” nature (Strait et al., 2013). These criticisms come despite the fact that Teaford and colleagues (Teaford, 1988a; Teaford & Glander, 1991, 1996; Teaford & Lytle, 1996; Teaford & Tylenda, 1991) have demonstrated that microwear turnover rates are associated with diet and different means of food preparation. Another criticism is the contribution of nonfood items such as abrasives, and nonmasticatory wear to the microwear signature (Wood, 2013). The argument is that the signature may reflect these behavioral aspects more than those of diet. While these are indeed circumstances to consider in analysis, that can and do contribute to the microwear signal, these issues can be combatted with robust sample sizes and ethnographic information (when available). Moreover, these aspects also should be seen as beneficial to our understanding of nuances in modern human dietary strategies.
Teaford and Glander (1991, 1996) found that microwear turnover happens within a few weeks, meaning that dental microwear signatures reflect a relatively short time before an individual's death. But, the speed of this turnover is patterned; it is a direct result of what was being consumed. For example, during the dry season mantled howling monkeys consumed new leaves, flowers, and green fruit, while during the wet season they ate more mature leaves (Teaford & Glander, 1991, p. 439). In the end, their work determined that microwear could elucidate seasonal, microhabitat, and intergroup differences in diet. In fact, other researchers have determined that dental microwear analysis, be it scanning electron microscope (SEM)-based, mesowear, or DMTA-based, can successfully distinguished diets of living animals well beyond primates and hominins, including hyraxes, carnivorous mammals, peccaries, rodents, rabbits, ungulates, and fish (e.g., Burgman, Leichliter, Avenant, & Ungar, 2016; Calandra et al., 2012; Caporale & Ungar, 2016; DeSantis, Schubert, Scott, & Ungar, 2012; Hoffman et al., 2015; Merceron et al., 2016; Merceron, Schulz, Kordos, & Kaiser, 2007; Purnell & Darras, 2016; Schmidt, 2008; Schulz et al., 2013; Scott et al., 2012; Solounias & Semprebon, 2002; Stynder, Ungar, Scott, & Schubert, 2012; Walker, Hoeck, & Perez, 1978).
Moreover, since microwear results from the destruction of the enamel (albeit at a microscopic scale), it is fortuitous that it changes dynamically; otherwise, it would indicate tooth wear no more precisely than does macrowear, which reflects a lifetime of masticatory and nonmasticatory usage. By constantly turning over, microwear provides an updated record of tooth use.
Another issue that arises, particularly with humans, is the concern that nonmasticatory wear can obscure masticatory wear. Nonmasticatory wear is created via many behaviors such as using teeth as tools to manipulate hide, sinew, cordage and other materials; habitually holding nonfood items like pipe stems or sewing needles with their teeth; and wearing facial piercings such as labrets (Alt & Pichler, 1998; Krueger, 2015, 2016b; Krueger et al., 2017; Krueger & Ungar, 2010, 2012; Milner & Larsen, 1991; Stojanowski, Johnson, Paul, & Carver, 2016).
Importantly, most dental wear caused by nonmasticatory behaviors occurs on the anterior dentition and can be readily differentiated from masticatory wear (see Teaford & Oyen, 1989; Teaford, 1991 for reviews. Also see Leigh, 1925; Molnar, 1971, 1972; Pedersen, 1947; and Taylor, 1963 for additional examples of unusual wear). For instance, large parallel scratches are commonly associated with striations from nonmasticatory behavior, whereas masticatory wear creates both large and fine scratches that intersect at acute angles (e.g., Krueger & Ungar, 2012). Parr (2012) reports labial modification of the incisor teeth in Guam; these modifications create sizable macroscopic wear feature that clearly are not related to ingestion or mastication.
Other wear features on molar teeth, such as “notches” (Bonfiglioli, Mariotti, Facchini, Belcastro, & Condemi, 2004) or “para-facets” (Fiorenza & Kullmer, 2013), are attributed to nonmasticatory behaviors, but are easily differentiated from masticatory wear based on feature morphology and location. For example, individuals who place nondietary items into their mouths tend to hold those items between their cheeks and teeth, generating buccal wear (Indriati & Buikstra, 2001) or wear on the anterior dentition (Lukacs & Pastor, 1988). As nonmasticatory wear features are easily identified, DMTA analysts can either digitally remove them or exclude the individual from analysis altogether.
A final concern regarding ancient human microwear is that DMTA was developed for interspecific comparisons. Nonetheless, microwear analysis has been employed in a number of intraspecific studies as well (Casserly, Van Sessen, & Schmidt, 2014; Chiu, Schmidt, Mahoney, & McKinley, 2012; El Zaatari, 2008, 2010; El Zaatari & Hublin, 2014; El Zaatari & Teaford, 2014; Estalrrich, El Zaatari, & Rosas, 2017; Estalrrich & Rosas, 2015; Karriger et al., 2016; Krueger, 2015; Krueger & Ungar, 2010; Larsen et al., 2001; Ma & Teaford, 2010; Mahoney et al., 2016; Organ, Teaford, & Larsen, 2005; Schmidt et al., 2016; Schmidt & Remy, 2016; Teaford & Robinson, 1989; Williams et al., 2018). An intraspecific study of humans is a challenge because human dietary strategies tend to overlap, even among people with disparate subsistence strategies. No matter how people define their food attainment strategies, people tend to eat items such as nuts, seeds, grasses, meat, and fish. Thus, although archeologists often categorize human groups into distinct subsistence strategies, it is clear that human subsistence patterns are not discrete entities. For example, ethnographic studies indicate that people in certain farming communities often forage in addition to rearing crops; this is the case in highland New Guinea where people both raise and collect wild yams as well as engage in animal husbandry (Strathern, 1975). Moreover, farmers who live near pastoralists, like those of Mongolia and northern China, are likely to trade with them and consume pastoral goods, and vice versa (e.g., Honeychurch, 2014).
For these reasons, the use of the terms forager, farmer, and pastoralist should not imply that this study considers human dietary endeavors to be discrete; rather, these terms serve to organize populations employing similar, although not identical, means of subsistence as defined by their respective archeological records. It should also be noted that human populations that differ in their subsistence strategies are not equivalent to, for example, different species of monkeys adapted to different diets. The humans studied here represent a single species with meaningful but largely subtle, locally- or regionally-based distinctions in food acquisition, preparation, and consumption.
4 FORAGER, FARMER, PASTORALIST DIET, AND SUBSISTENCE
The term “diet” is meant to represent the food that is actually consumed, whereas “subsistence” represents both the diet and the behaviors necessary to acquire food (e.g., Hillson, 1979). Foragers, are those groups that gather, hunt, and/or fish for their sustenance. This aggregate of wild food acquisition strategies often, but not always, leads to mobile groups having limited material culture related to food processing; that is, they usually do not have heavy grinding stones or grinding wheels to process the wild plants they consume and, for the most part, their food processing is limited to cooking. Some foragers eat high levels of meat, which have microwear signatures indicative of a softer diet since meat, itself, does not affect the teeth (El Zaatari, 2008; Hua et al., 2015; Karriger et al., 2016). Others eat predominantly tough and fibrous foods and/or hard foods like seeds and nuts that lead to numerous sizable microwear features (Schmidt, 2001; Schmidt, 2010). Farmers, on the other hand, consume domesticated plants, most frequently grains; but other early domesticates included tubers, cucurbits, drupes, and leafy plants (e.g., Johannessen, 1993; Lebot, 1999; Perrier et al., 2009; Zeder, 2011).
Farmers tend to be more sedentary and over the millennia developed sophisticated means of processing their foods, including using well-made ceramics that could sustain a boil and elaborate grinding methods to grind their grains. Thus, they often have far softer diets than their foraging counterparts (e.g., Schmidt et al., 2016). However, plants chosen for domestication have high a carbohydrate content, including disaccharides, which are highly cariogenic. While their teeth may not have been worn down as quickly as those of foragers, it is common for farmers to have higher levels of oral pathology, particularly dental caries (Watson, 2008; see Larsen, 2015 for a comprehensive review). Early pastoralists were usually mobile people who focused on animal husbandry. Despite their mobility, they were capable of amassing sizable quantities of material culture because of their utilization of work animals. Moreover, their transhumance lent itself to meeting other populations, particularly farming groups with whom they often traded (Honeychurch, 2014; Machicek & Zubova, 2012; Makarewicz, 2011). Pastoral dietary staples, such as meat, cheese, and yoghurt, tended to be soft. But, the foods for which they traded, like grains, could have been stone-ground and capable of producing low to moderate levels of microwear (Schmidt et al., 2016). The diets and/or subsistence patterns for the specific groups used herein are provided in the Methods section.
5 RESEARCH HYPOTHESES
It is clear at this point that DMTA can help with paleodietary reconstructions of humans in particular groups or populations. What has not yet been determined is DMTA's efficacy with a global distribution of populations. The current project's goal is to determine if DMTA can detect microwear differences in large samples of foragers, farmers, and pastoralists from both Old and New World locales in an effort to determine if the aforementioned dietary regimes can be distinguished statistically. It focuses on two DMTA variables: complexity, which represents surface coarseness, and anisotropy, which represents similarity of feature orientation. The hypotheses for this study are tested parametrically via analysis of variance (ANOVA) and are explained in detail below. A follow-up K-means cluster analysis is undertaken to explore relationships between the locales that make up each subsistence group.
Three research hypotheses are explored. The first hypothesis, H1, is that the texture variables complexity and anisotropy will distinguish foragers, farmers, and pastoralists. Based upon the findings mentioned above, it is expected that foragers will have greater complexity and lower anisotropy values than the others related primarily to their less processed diets of harder foods like seeds and nuts. The pastoralists should have the lowest complexity, because they tend to have the highest proportion of meat and/or milk and cheese in the diet. The farmers should have the highest anisotropy because their rather homogenous diets generate consistent jaw movements. Because the farmer and forager samples are geographically and temporally diverse, two additional hypotheses are addressed.
The second, H2, is that complexity and anisotropy will differentiate Old World (OW) and New World (NW) foragers and farmers. The thought here is that the NW farmer diet, which was almost exclusively maize, might have elevated complexity values compared to that of the OW farmers, which exploited an array of agricultural goods. In particular, European farmers were helped by animals like oxen capable of turning large grinding stones and producing fine flours. In contrast, NW farmers had no beasts of burden and processed their grain manually, primarily via stone tools such as manos and metates (see Benz, 2009). Thus, the NW diet may have included less refined foods and/or more exogenous grit because of its more modest means of processing.
The third hypothesis, H3, states that complexity and anisotropy will differentiate Early and Late OW farmers. It addresses the sizable temporal range of the OW farmers by dividing them into Early (Neolithic and the Early Bronze Age) and Late (Late Bronze Age to medieval period) groups. This division is based on technological shifts during the late Bronze Age that improved food processing thereafter, such as the use of less abrasive grinding stones (e.g., Roman basalt querns) and boiling facilitated by improved ceramics (e.g., Barker, 1985). This hypothesis, therefore, is similar to H2 in that it considers technological differences related to food processing, with the premise that improved processing leads to less complex microwear textures. An inverse relationship between dietary abrasiveness and food processing has been demonstrated by way of dental macrowear study. For the most part, macrowear has decreased through time in human populations as food processing techniques have improved (e.g., Molnar, 1971, 1972; Molnar, McKee, Molnar, & Przybeck, 1983; Schmidt 1998; Watson, 2008; Schmidt, 2010). Moreover, Schmidt (2010) found in an SEM-based study that wider microwear scratches were more common in populations with greater macrowear and that macrowear decreased through time as scratch widths decreased. Thus, it is expected here that microwear texture signatures will indicate coarser diets for the Early OW people who had comparatively less effective means of food processing.
The null hypotheses, H0, for the ANOVA tests are:
H1
H01: There is no statistically significant difference in complexity based upon dietary group (forager, farmer, pastoralist)
H02: There is no statistically significant difference in anisotropy based upon dietary group (forager, farmer, pastoralist)
H2
H03: There is no statistically significant difference in complexity based upon location (Old World, New World)
H04: There is no statistically significant difference in anisotropy based upon location (Old World, New World)
H3
H05: There is no statistically significant difference in complexity based upon time among the Old World sample (Early, Late)
H06: There is no statistically significant difference in anisotropy based upon time among the Old World sample (Early, Late)
Because null hypothesis significance tests (NHST) are considered by some to be insufficient indicators of the relationships of variables (Rosnow & Rosenthal, 2009; Smith, 2018) we also include 95% confidence intervals and effect sizes in our results. This aspect of the study is described in more detail below.
6 MATERIALS
This study includes data from 719 individuals from 26 locales (representing 51 archeological sites) from North and South America, Europe, Asia, Africa, and Australia (see Figure 1 and Table 1). In total, the sample includes 450 farmers, 192 foragers, and 77 pastoralists. Most of the sites have excellent archeological records, which were used to summarize their subsistence and dietary patterns.
| Location | Sites | Temporal assignment | N |
|---|---|---|---|
| Foragers | |||
| 1.Australia | Gillman, Yorke peninsula | 1,100–600 BP, precontact | 10 |
| 2. Atacama, Chile | Morro 1, Acha 3 | ~7,000 BP | 7 |
| 3. Lagoa Santa, Brazil | Harold Walter, Cerca Grande, Lapa das Carrancas, Santana do Riacho, Lapa Vermelha IV, Lapa do Santo | 11,000–7,000 BP | 21 |
| 4. Czech Republic, mid-upper Paleolithic | Dolní Vĕstonice | ~27,000–25,000 BP | 6 |
| 5. Israel | Ohalo 2 | 23,500–22,500 BP | 1 |
| 6. Israel, Natufian | Ein Mallaha, Nahal Oren, Hayonim, Rakefet | 14,000–11,000 BP | 19 |
| 7. Laos | Tam hang | 13,740 ± 80BP/Neolithic | 7 |
| 8. Niger | Gobero | 10,000–4,000 BP | 10 |
| 9. US, Indiana, Kentucky (archaic) | Barrett, bluegrass, Butterfield, Carlston Annis, Chiggerville, Indian knoll, Kramer, Meyer, ward | Middle to late archaic, 6,000–3,000 BP | 78 |
| 10. US, Indiana (middle woodland) | Bicycle bridge, Mann, New Castle, white, Windsor | ~2,000 BP | 33 |
| Forager Total | 192 | ||
| Farmers | |||
| 11. England, medieval | Canterbury | ~700–500 BP | 27 |
| 12. Peru | Cerro Cerillos, Huaca Sialupe | Middle Sicán period 1,100–900 BP | 15 |
| 13. Egypt | Egypt: Hierakonpolis, Giza | Predynastic period approx.. 6,500–5,150 BP; old kingdom 4,700–4,190 BP | 45 |
| 14. Greece | Mitrou, Tragana Agia Triada | Late bronze and early iron age 3,600–3,000 BP | 16 |
| 15. England, early bronze, late bronze, and iron age | Amesbury, Boscombe, Early's farm, Fighel dean, Norton Bavant Barrow, Shrewton, Stonehenge | Early bronze, late bronze, iron age, ~4,500–3,000 BP | 44 |
| 16. Peru, late | Gentilar | ~400 BP | 11 |
| 17. Italy, Roman | Herculaneum | 1,871 BP (AD 79) | 58 |
| 18. Iraq | Kish | ~5,000 BP | 55 |
| 19. Mexico | La playa | 3,000–2,000 BP | 16 |
| 20. Indiana, Ohio, (late woodland/ Ft. ancient) | Taylor mound, ray, woodland ridge | ~700 BP | 23 |
| 21. Nepal | Mebrak & Sam Dzong | ~2,400–1,400 BP | 18 |
| 22. US, Indiana, Illinois (Mississippian) | Angel, Orendorf | ~600 BP | 35 |
| 23. Israel, Neolithic | Abu gosh, Atlit yam, Horvat Galil, Kfar Hahoresh | ~8,000 BP | 22 |
| 24. Southern Levant | Tell Dothan | Bronze and iron ages 3,500–2,700 BP | 51 |
| 25. Sudan | Tombos | New kingdom period (~3,550–3,070 BP), to the Napatan periods (~3,070–2,664 BP) | 14 |
| Farmer Total | 450 | ||
| Pastoralists | |||
| 26. Mongolia | Various late bronze and early iron age and Xiongnu sites including Eiigen Gol | 3,200–1,850 BP | 77 |
| TOTAL: | 719 |
The foragers are from North and South America, Africa, Europe, Asia, and Australia. The North American foragers include Middle/Late Archaic-period (6,000–3,000 BP) terrestrial and riverine foragers and Early/Middle Woodland-period (3,000 BP – AD 500) forager-horticulturalists. The Archaic populations inhabited areas along the Ohio and Green Rivers in Indiana and Kentucky, and collected mussels and fish as well as terrestrial plants and nuts, particularly hickory (Jefferies, 2009; Yarnell, 1993). The Early and Middle Woodland people are from the same general area and foraged as well; they focused heavily on tree nut consumption, but also engaged in low-level horticulture of starchy and oily seeds such as Chenopodium and knotweed (Polygonum) (Ford, 1979; Fritz, 1993; Gremillion, 1996; Gremillion & Sobolik, 1996). The South American foragers include people from Lagoa Santa in Central-Eastern Brazil, who were paleo-American foragers dating 11,000 to 7,000 BP. They were terrestrial foragers with subsistence based on middle to small-sized animals, such as deer, armadillos, peccaries, cavies, birds, fishes, reptiles, amphibians, and mollusks, but they also relied heavily on plant sources like wild tubers and fruits (Bernardo, Neves, & Kipnis, 2017; Da-Gloria & Larsen, 2014, 2017). The Archaic period (10,000–3,500 BP) Chinchorro people from the Atacama region of Chile (Morro 1 and Acha 3 sites) primarily exploited marine resources but occasionally consumed plants from the Andean foothills to their east (Arriazza, 1995; Arriazza, Doubrava, Standen, & Haas, 2005). The African foragers are located in Niger and date from 9,000 to 5,000 BP. They likely subsisted as fishers as well as terrestrial foragers (Sereno et al., 2009). The European foragers are from the Mid-Upper Paleolithic site of Dolní Vĕstonice (27,000–25,000 BP). They consumed small to large-bodied animals and locally available plant foods (e.g., el Zaatari and Hublin 2014; Power, Salazar-García, & Henry, 2016; Wilczyński, Wojtal, Robličková, & Oliva, 2015; Wojtal & Wilczyński, 2015). The foragers from Asia include groups from Israel and Laos. The population from Israel consists of one individual from Ohalo 2 (23,500–22,500 BP) and Natufian people who date from 14,000 to 11,000 BP; they primarily consumed wild grains (Bar-Yosef, 1998; Bar-Yosef & Meadow, 1995; Hopf & Bar-Yosef, 1987). The group from Laos (which may have some Neolithic components) dates to around 13,740 BP (Willman, Shackelford, & Demeter, 2016). The foragers from Australia date to 1,100–600 BP. They are primarily inland foragers who likely consumed kangaroos, dogs, emus, lizards, shellfish, and fish, as well as wild plants including fruit, seeds, and grasses (Littleton & Scott, 2016).
The farmers come from North and South America, Africa, Europe, and Asia. The North American farmers include Mississippian (800–600 BP) people from Indiana and Illinois and farmers from Mexico who date to about 2,000 BP. The South American farmers include people from the Middle Sicán period (~1,100–900 BP) of Peru as well as early contact-era (~400 BP) people who also are from Peru. All of the farmers from the Americas focused on maize production, although they likely supplemented their diets with wild and horticultural goods (e.g., Benz, 2009; Bush, 2004; Staller & Carrasco, 2009). The African farmers come from the Predynastic and Old Kingdom periods (6,500–4,190 BP) of Egypt and from the New Kingdom Period (~1,550–1,070 BP) to Napatan Period (~1,070–664 BCE) Nubian site of Tombos in northern Sudan (Buzon, 2014; Buzon, Smith, & Simonetti, 2016). The groups from Europe include Early Bronze to Iron Age (4,500–3,000 BP) and Medieval (800–500 BP) people from England; Late Bronze to Early Iron Age people from Greece (3,600–3,000 BP); and Roman-era people from Italy (1,871 BP). The Asian farmers include Neolithic groups from Israel (~8,000 BP); Bronze and Iron Age people from the Southern Levant (3,261–2,973 BP (Gregoricka & Sheridan, 2018)), Iraq (~5,000–2,700 BP), and Nepal (~2,400–1,400 BP). In general, the farmers from Europe, Asia, and Africa (i.e., the Old World) focused on wheat farming, although other crops were grown as well (Willcox, 1998, 1999). The groups in England and Sudan grew Emmer wheat. Other products included spelt wheat, and six-row barley (Barker, 1985). The Greek and Roman economies were very complex and included domesticated and wild plants and animals. The Romans of Herculaneum had access to a wide array of foods including wheat, barley, oats, wild nuts, local and exotic meats, and figs (Robinson & Rowan, 2015). The farmers from Nepal focused on buckwheat (Knörzer, 2000).
The pastoralist sample includes Xiongnu period and Late Bronze Age/Early Iron Age people from Mongolia and date from between 3,200 and 1,850 BP. They appear to have consumed meat, milk, and yoghurt as well as millet they acquired through trade with nearby farming groups (Makarewicz, 2011).
All individuals in the current study have only adult teeth and no individual is younger than approximately 12–18 years old. Deciduous teeth produce microwear that is suitable for study (e.g., Bullington, 1991; Kelly, 2018; Krueger, 2016a; Mahoney et al., 2016; Remy, Schmidt, D'Anastasio, & Reinhardt, 2014), but they are less mineralized than their adult counterparts (Wilson & Beynon, 1989). Moreover, Darnell and colleagues (2010) determined that mineralization can affect enamel's mechanical properties. The current study, therefore, excludes deciduous teeth in an effort to control for enamel composition.
Age determinations followed osteological standards (e.g., Buikstra & Ubelaker, 1994). The youngest person in the study comes from Lagoa Santa and is approximately 12 years old. The remainder are primarily young and middle adults (i.e., 18–50). Old adults (those thought to be over 50) are excluded because their teeth tend to be too worn for DMTA. The sexes are combined because sex-based differences are uncommon in comparisons of intra-populational variation in humans (e.g., Schmidt et al., 2016). There is a reported sex-based difference in DMTA for the El Sidrón Neandertals (Estalrrich & Rosas, 2015) and a minor difference at Herculaneum (Remy et al., 2014). Beyond these two examples, however, human male and female DMTA values tend to be the same.
7 METHODS
Data collection followed standard procedures for DMTA (e.g., Scott et al., 2006). Most of the dental molding took place at the facilities housing the collections. Casting, on the other hand, usually took place at the University of Indianapolis (UIndy) Bioarchaeology Laboratory. Molding required teeth to be cleaned with alcohol (usually 95% ETOH) and a cotton swab. The molding agent used was Coltene's President Jet, light body; the casting material was Super Hard Epoxy Resin®. Buikstra and Ubelaker (1994) recommended these materials, which have a long history of successful replica-making. A recent test of impression material efficacy found President's Jet to be superior to other commercially available options (Goodall, Darras, & Purnell, 2015).
Dental replicas were viewed via a Solarius Sensofar Plμ® white-light confocal profiler (WLCP) housed at UIndy. For each individual a single maxillary or mandibular first or second molar was studied; in total the study included 568 mandibular and 151 maxillary molars. Preliminary observations were made at 10X magnification in order to find unobscured areas of interest within facet 9. Facet 9 is a standard location for DMTA study (see Scott et al., 2006). It usually appears as a worn region between the buccal cusps on mandibular molars and between lingual cusps on the maxillary molars, which forms during Phase II of the power stroke of the chewing cycle (Hiiemae & Crompton, 1985; Meier & Schneck, 1982). Data were collected using a 100x Nikon extra-long working distance (ELWD) objective lens from four contiguous areas that were automatically stitched together; the total area studied was approximately 242 x 182 μm. Data point spacing was 0.17 μm in the x-y plane and 0.20 μm in the z plane.
Data from each specimen were imported into SolarMap® (version 5.1.1), which was used to level the data (using the least squares leveling algorithm) and to digitally “clean” areas unsuitable for study. Cleaning is an important step since it removes adherent particles and debris and any nonmasticatory wear from the dataset. To keep surface sizes from each specimen similar, suitable specimens had cleaned areas that were no more than 10% of the total surface area. The authors closely inspected data clouds and viewed them as both 2D photosimulations (which are visual representations of the data that emulate SEM micrographs) and as 3D representations. This inspection process served as a critical means to ensure that only appropriate surfaces expressing true diet-driven microwear features were included; those obscured by consolidants or other fine films were excluded (e.g., Teaford, 1988b).
The data clouds were imported into scale-sensitive fractal analysis software to calculate surface characteristics (see Scott et al., 2005; Scott et al., 2006). In Sfrax®, each file was given a 5% valley suppression and saved as an. SDF file. The. SDFs were imported into Toothfrax® for complexity and anisotropy calculations. These procedures are largely identical to those for other DMTA labs, although the valley suppression is an adjustment meant to calibrate the WLCP at UIndy with the original profiler at the University of Arkansas and to ensure cleaned areas were excluded from surface calculations. It is important to have such calibrations to ensure that data collection is standard across different profilers (e.g., Arman et al., 2016).
The current study focuses on two texture variables shown to be especially useful in discerning dietary strategies: area-scale fractal complexity (Asfc), and exact-proportion length-scale anisotropy of relief (epLsar 1.8). Complexity output is the steepest slope of a curve on a log–log plot of relative surface area versus scale in microns squared, multiplied by −1,000 (see Scott et al., 2006 for more details regarding this calculation). More complex structures, such as those with higher complexity values, are those that are coarser and appear rough (often pitted) when viewed microscopically. In humans from archeological contexts, surface complexities tend to range from 0.5 to 3, with most groups between 1 and 2 (e.g., Chiu et al., 2012; Frazer, 2012; Mahoney et al., 2016; Remy et al., 2014; Van Sessen, Schmidt, Sheridan, Ullinger, & Grohovsky, 2013), although higher values have been reported (e.g., El Zaatari, 2010). Nonhuman complexities may exceed 6 (e.g., DeSantis, 2016).
Anisotropy measures feature orientation; high anisotropy values indicate features are oriented in a common direction. It is calculated by dividing 36 length vectors, separated in 5° intervals, by the sum of all other vectors and then computing a mean for each individual (i.e., the exact proportion method; see Scott et al., 2006). Consequently, output values are very small fractions and in humans tend to range between 0.0005 and 0.0090, with most populations averaging between 0.0020 and 0.0040. Anisotropy tends to indicate the degree to which the jaw moves in a consistent direction. Tough fibrous foods tend to generate higher anisotropy values, while harder diets tend to generate lower values (e.g., Chiu et al., 2012; El Zaatari, 2010; Frazer, 2012).
Statistical tests included univariate ANOVA. This test provides many advantages, including maintaining its robustness when the data deviate from a normal distribution, and when variances differ and sample sizes are uneven (Sokal & Rohlf, 1995). Nonetheless, normality and variance equality were tested using Shapiro–Wilks' and Levene's tests, respectively. The data were rank transformed in instances where the assumption violations were too great. Because of their impact on the data, outliers beyond three times the interquartile range were removed; therefore the sample sizes differ for each variable. All told, six univariate ANOVA tests were run with complexity and anisotropy serving as the dependent variables. The independent variables were fixed factors determined by the research hypothesis. Thus, for H1, the fixed factor was dietary group (e.g., forager, farmer, and pastoralist). For H2 the fixed factor was location (e.g., OW and NW). For H3 the fixed factor was time (e.g., Early and Late farmer). The post hoc test used was Fisher's least significant difference (LSD), which is meant to elucidate subtle differences.
Recent criticisms of null hypothesis statistical tests (NHST) point out that null hypothesis testing is misused in the sciences, including biological anthropology (Ferguson, 2009; Smith, 2018). Critics argue that measures of effects and effect confidence intervals (CIs) are neglected statistical indicators of relationships that are superior to p-values because the latter are affected by sample size. Moreover, at times NHST-based studies lack a true null condition; there exists no a priori reason to expect the test groups to have identical means (e.g., Ferguson, 2009; Rosnow & Rosenthal, 2009; Tukey, 1991). What measures of effect provide are succinct indications of the relationship between variables; higher effect values with smaller CI's indicate stronger effects. Moreover, the CI provides an indication of the significance of the relationship. For some effect indicators, if the CI is large enough to encompass 0, it is thought to represent a weak, nonsignificant relationship. If it does not include 0, then a significant difference is indicated. Thus, there is a growing number of social scientists who believe that effect sizes and their CI's can replace NHST (see Smith, 2018). There are several effect indicators available to analysts, many of which are already familiar to anthropologists; for example, r2 is commonly used for understanding linear relationships.
The current study does not abandon the use of NHST, but it does couple NHST with effect indicators and their CI's. Employing NHST is valid in this case because the study has groups that could have statistically similar microwear values, for example, a null condition. But, this study has large sample sizes that could inflate p-values; therefore, the effect indicators are important aids when interpreting microwear and diet relationships. If, for example, the NHST indicates a significant difference, but the effect indicators show that the effect is extremely minor, then it is possible that a Type I error occurred related to sample size. But, if the effect indicators indicate an intermediate or strong relationship, then the NHST results are likely not spurious. In the end, the effect indicators and their confidence intervals help to validate the NHST.
The indicator of effect size is partial eta squared (η2 p) for comparisons that include more than two groups. Comparisons made between two groups used Hedges's g. Partial eta squared values around 0.01 are considered small. Values at or around 0.09 are considered to have a medium effect and values at or above 0.25 are considered large effects. For Hedges g, small, medium, and large effect sizes are 0.2, 0.5, and 0.8, respectively. Effects 95% CIs were determined using an online calculator (https://effect-size-calculator.herokuapp.com/). For the ANOVAs, alpha values were set at 0.05. Finally, a follow-up K-means cluster analysis was performed to see how the populations (organized into 26 Locales [recall Table 1]) sort out by microwear signature. This analysis used standardized z-scores of the means for complexity and anisotropy for each Locale and were sorted into three clusters, since the study involves three over-arching subsistence strategies. For all tests, computations were made via SPSS 25.
8 RESULTS
The Levene's and Shapiro–Wilk tests indicated the test groups for the ANOVAs had significantly different variances and nonnormal distributions (just three tests indicated equal variances: anisotropy for H2, and both complexity and anisotropy for H3). However, it was determined that in order to maintain the greatest test power, only the comparisons made for H1 required a rank transformation, because it had both parameters fail to meet ANOVA assumptions. For the remaining tests, the standard ANOVA was less likely to generate Type I or Type II errors than transformations or nonparametric tests given (a) the large sample sizes, (b) the fact that for each test the variances were within 1.5 times of each other, and (c) that the distributions were modestly skewed to the right and not bimodal or platykurtic (e.g., Glass, Peckham, & Sanders, 1972; Moore & McCabe, 2003; Sokal & Rohlf, 1995).
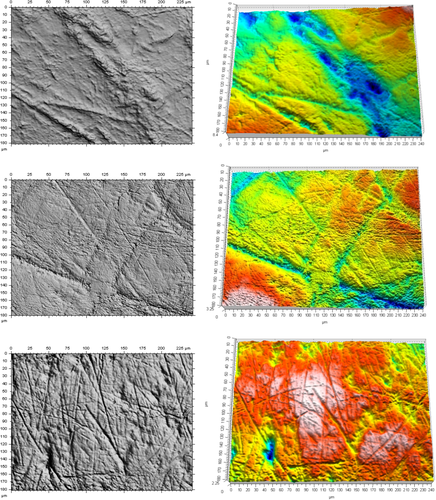
Descriptive statistics and ANOVA results are presented in Tables 2-7, and representative 2D and 3D images are presented in Figure 2. The mean summary data indicate that complexity decreases from foragers (1.47) to farmers (1.36) to pastoralists (0.89), with pastoralists having much lower Asfc values than the other groups (Table 2). There are, however, some farming individuals from North America whose molar microwear is of high complexity. These individuals come from the Late Woodland Ray site, Fort Ancient Taylor Mound, and the Mississippian Orendorf site, which are roughly contemporaneous maize consuming groups from the Ohio River Valley. Their very high complexities are unusual for farmers, particularly since their caries and dental health indicators are consistent with maize agriculture (e.g., Raypole & Schmidt, 2012; Schmidt & Greene, 2003). A review of the literature found that these findings were consistent with the subsistence and isotopic records for late precontact inhabitants of the midcontinent, which indicate a marked nut consumption (e.g., Emerson, Hedman, & Simon, 2005) and somewhat mitigated stable carbon isotope values when compared to other maize farmers (e.g., Cook & Schurr, 2009). This determination required that these groups be removed from the H1 tests because they represented both farming and foraging groups (i.e., a mixed economy).
| Complexity (Asfc) and anisotropy (epLsar 1.8) by subsistence group | ||||
|---|---|---|---|---|
| Complexity (Asfc) | Mean | SD | 95%CI of Mean | N |
| Foragers | 1.47 | 0.578 | 1.38–1.55 | 168 |
| Farmers | 1.36 | 0.566 | 1.29–1.40 | 385 |
| Pastoralists | 0.89 | 0.304 | 0.764–1.02 | 70 |
| Total | 623 | |||
| Anisotropy (epLsar1.8) | Mean | SD | 95% CI of Mean | N |
| Foragers | 0.0027 | 0.0013 | 0.0025–0.0028 | 181 |
| Farmers | 0.0035 | 0.0018 | 0.0033–0.0037 | 392 |
| Pastoralists | 0.0034 | 0.0015 | 0.0030–0.0038 | 63 |
| Total | 636 | |||
| Rank transformed complexity and anisotropy | |||||
|---|---|---|---|---|---|
| Complexity (Asfc) | df | F | Sig. | η2p | η2p CI |
| Foragers vs Farmers | 2 | 31.823 | <0.000 | 0.095 | 0.060–0.131 |
| vs Pastoralists | |||||
| LSD Post hoc | Sig. | ||||
| Foragers vs Farmers | 0.032 | ||||
| Foragers vs Pastoralists | <0.000 | ||||
| Farmers vs Pastoralists | <0.000 | ||||
| Anisotropy (epLsar1.8) | df | F | Sig. | η2p | η2p CI |
| Foragers vs Farmers | 2 | 14.031 | <0.000 | 0.043 | 0.019–0.070 |
| Vs Pastoralists | |||||
| LSD Post hoc | Sig. | ||||
| Foragers vs Farmers | <0.000 | ||||
| Foragers vs Pastoralists | 0.001 | ||||
| Farmers vs Pastoralists | 0.986 | ||||
| Complexity (Asfc) and anisotropy (epLsar 1.8) by Old World (OW) and New World (NW) foragers and farmers | ||||
|---|---|---|---|---|
| Complexity (Asfc) | Mean | SD | 95% CI of Mean | N |
| Foragers | 172 | |||
| OW | 1.55 | 0.679 | 1.34–1.75 | 46 |
| NW | 1.45 | 0.562 | 1.35–1.55 | 126 |
| Farmers | 428 | |||
| OW | 1.39 | 0.574 | 1.34–1.46 | 340 |
| NW | 1.41 | 0.646 | 1.27–1.54 | 88 |
| Total | 600 | |||
| Anisotropy (epLsar1.8) | Mean | SD | 95% CI of Mean | N |
| Foragers | 185 | |||
| OW | With Natufians | |||
| 0.0030 | 0.0015 | 0.0029–0.0036 | 49 | |
| Without Natufians | ||||
| 0.0027 | 0.0014 | 0.0022–0.0032 | 34 | |
| NW | 0.0026 | 0.0013 | 0.0024–0.0028 | 136 |
| Farmers | 432 | |||
| OW | 0.0034 | 0.0017 | 0.0032–0.0036 | 339 |
| NW | 0.0033 | 0.0016 | 0.0030–0.0036 | 93 |
| Total | 613 | |||
| Complexity and anisotropy for Old World (OW) vs. New World (NW) with and without Natufians | |||||
|---|---|---|---|---|---|
| Complexity (Asfc) | df | F | Sig. | Hedges's g | Hedges's g CI |
| OW Foragers vs NW Foragers | 1 | 0.809 | 0.370 | 0.167 | −0.170–0.506 |
| OW Farmers vs NW Farmers | 1 | 0.013 | 0.910 | −0.034 | −0.270–0.201 |
| Anisotropy (epLsar 1.8) | df | F | Sig. | Hedges's g | Hedges's g CI |
| OW Foragers vs NW Foragers | |||||
| With the Natufians | 1 | 4.255 | 0.041 | 0.293 | −0.033–0.623 |
| Without the Natufians | 1 | 0.092 | 0.762 | 0.075 | −0.300–0.452 |
| OW Farmers vs NW Farmers | 1 | 0.512 | 0.475 | 0.060 | −0.170–0.290 |
| Summary data for early and late farmers (all from OW) Complexity (Asfc) and anisotropy (epLsar 1.8) by early and late farmers | ||||
|---|---|---|---|---|
| Complexity (Asfc) | Mean | SD | 95% CI of Mean | N |
| Early farmers | 1.53 | 0.585 | 1.42–1.63 | 114 |
| Late farmers | 1.33 | 0.572 | 1.25–1.41 | 202 |
| Total | 316 | |||
| Anisotropy (epLsar1.8) | Mean | SD | 95% CI of Mean | N |
| Early Farmers | 0.0036 | 0.0017 | 0.0033–0.0038 | 117 |
| Late Farmers | 0.0034 | 0.0018 | 0.0031–0.0036 | 205 |
| Total: | 322 | |||
| ANOVA complexity (Asfc) and anisotropy (epLsar 1.8) for early vs. late farmers | |||||
|---|---|---|---|---|---|
| Complexity (Asfc) | df | F | Sig. | Hedges's g | Hedges's g CI |
| Early vs. Late farmers | 1 | 8.029 | 0.005 | 0.346 | 0.115–0.578 |
| Anisotropy (epLsar 1.8) | df | F | Sig. | Hedges's g | Hedges g CI |
| Early vs. late farmers | 1 | 1.218 | 0.271 | 0.059 | -0.169–0.286 |
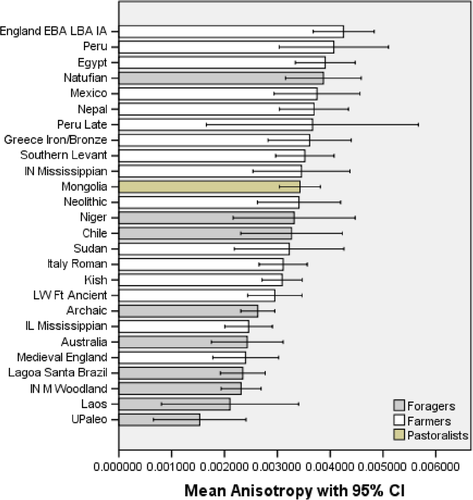
The anisotropy summary data indicate that farmers and pastoralists share nearly identical mean values (0.0035 and 0.0034, respectively), while the foragers have a lower mean value (0.0027; Figure 3, Table 2). Upon closer inspection, the data indicate that most farmers have epLsar values above 0.0030, except those with mixed economies. By contrast, foragers tend to have values that range between 0.0020 and 0.0030. The Natufians were the lone forager group to have an anisotropy high enough to place it near the farmers (Figure 4).
8.1 H1 (foragers vs. farmers vs. pastoralists)
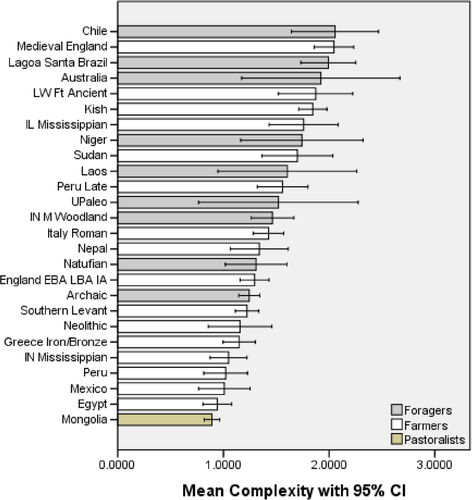
The ANOVAs for complexity and anisotropy found statistically significant differences for the foragers, farmers, and pastoralists. The complexity post hoc results indicate significant differences between all three subsistence categories (sig. = 0.032 for foragers vs. farmers and sig. <0.000 for foragers vs. pastoralists and farmers vs. pastoralists). For anisotropy, significant differences were found between foragers and farmers (sig. <0.000) and between foragers and pastoralists (sig. = 0.001), but not between farmers and pastoralists (sig. = 0.986). Thus, the null hypotheses (H01 and H02) for complexity and anisotropy are rejected. The effect size values for complexity and anisotropy were 0.085 and 0.047, respectively. The complexity effect value of 0.085 supports rejecting the null hypothesis because it indicates a medium effect. The anisotropy effect size of 0.047 is small to medium because a difference was not found between the foragers and farmers (see Tables 2 and 3). However, this inability to distinguish these two groups is notable and discussed later.
8.2 H2 (NW foragers vs. OW foragers; NW farmers vs. OW farmers)
The H2 results indicate a difference between NW and OW groups. It excluded pastoralists because no NW pastoral groups were included in the study. A significant difference was found between OW and NW foragers for anisotropy (sig. = 0.046), but if the Natufians are excluded from the forager group (because of their suspected incipient agricultural ways), the difference is no longer statistically significant. The Natufians were exaggerating the difference between the OW and NW groups. No differences were found between OW and NW farmers and no differences were found for complexity between OW and NW foragers. Thus, the original null hypothesis (H04) is rejected for anisotropy between the NW and OW foragers. The study fails to reject the other NW versus OW null hypotheses. The complexity Hedges g value for OW versus NW foragers was low at 0.167 and very low at −0.034 for OW versus NW farmers. The OW versus NW forager anisotropy Hedges g was high at 0.293 including the Natufians, but only 0.075 excluding the Natufians. Thus, the NW-OW forager difference in anisotropy is dependent on the inclusion of the farmer-like Natufians in the OW group. The OW versus NW farmer Hedges g was low 0.060 (see Tables 4 and 5).
8.3 H3 (early versus late OW farmers)
The H3 ANOVAs indicate a significant difference for complexity between the Early (Neolithic and Early Bronze Age) and Late OW farmers (Late Bronze Age through the Medieval Period), with higher values for the former farmers (sig. <0.000), but there was no significant difference for anisotropy. Thus the null hypothesis for complexity (H05) is rejected while the null for anisotropy failed to be rejected. The η2 p values are 0.346, for complexity (which is fairly high) and 0.059 for anisotropy, which is low (see Tables 6 and 7).
8.4 Post priori K-means cluster analysis
K-means cluster analysis convergence was achieved after three iterations. It sorted the population samples from the 26 Locales into clusters that, for the most part, distinguish foragers and farmers. The first cluster had members with low complexity and low anisotropy. It had five members and was dominated by foragers, although curiously it included the group from Herculaneum. The foragers included the Archaic, Indiana Middle Woodland, the Late Upper Paleolithic group from Laos, and the Mid-Upper Paleolithic. The second cluster included Locales with high complexity but low anisotropy. It included four foraging groups and five farming groups. The foragers include the Aboriginal Australians, the early Holocene population from Chile (the Chinchorro), the Lagoa Santa Paleoindians, and the middle Holocene group from Niger. The farmers included the mixed economy Illinois Mississippian and the Late Woodland/Ft. Ancient groups. It also included early farmers from Kish, the farming population from Sudan (Tombos), and Medieval farmers from England. The last cluster consisted of groups with low complexity but high anisotropy. It was dominated by farmers. In fact, the only foragers in this cluster were the Natufians. The others were the Egyptians, Early through Iron Age groups from England, Bronze, and Iron Age Greeks, Mississippians from Indiana, late farmers from Mexico, Neolithic groups from the Levant, farmers from Nepal, Inka and post-Inka groups from Peru, and farmers from Tell Dothan in the southern Levant. This cluster also included the pastoralists (see Table 8).
| Cluster 1 | Cluster 2 | Cluster 3 | |||
|---|---|---|---|---|---|
| Locale | Cluster distance | Locale | Cluster distance | Locale | Cluster distance |
| US, Indiana, Kentucky, archaica | 0.715 | Australiaa | 0.603 | Egyptb | 0.644 |
| US, Indiana, middle woodlanda | 0.049 | Atacama, Chilea | 0.805 | England, early bronze to iron ageb | 0.896 |
| Laosa | 0.544 | Illinois Mississippianc | 0.651 | Greeceb | 0.149 |
| Upper Paleolithica | 1.206 | Iraqb | 0.396 | US, Indiana, Illinois Mississippianb | 0.472 |
| Italy, Romanb | 1.144 | Lagoa Santa, Brazila | 0.783 | Mexicob | 0.401 |
| Indiana, Ohio, late woodland/Ft. ancientc | 0.180 | Mongoliad | 0.830 | ||
| England, Medievalb | 0.780 | Israel, Natufiana | 0.490 | ||
| Nigera | 0.814 | Israel, Neolithicb | 0.444 | ||
| Sudanb | 0.766 | Nepalb | 0.514 | ||
| Perub | 0.640 | ||||
| Peru, lateb | 1.118 | ||||
| Southern Levantb | 0.342 | ||||
- a Foragers.
- b Farmers.
- c Mixed economy farmers.
- d Pastoralists.
9 DISCUSSION
With regard to H1, the results indicate that the human subsistence categories used herein can be distinguished via DMTA. As seen in the overall complexity and anisotropy means, foragers generally have greater complexity and lower anisotropy compared to farmers and pastoralists. Farmers tend to have lower complexity and higher anisotropy than the foragers, but greater complexity than the pastoralists. The farmer and pastoralist anisotropy values are indistinguishable.
Foragers stand out with significantly higher complexity values relative to both farmer and pastoralist samples. Foragers tend to eat a range of foods that are harder and less processed, creating pitted, coarse occlusal surfaces (e.g., Schmidt, 2001). Farmers, on the other hand, often consume more processed foods that can be softer, but still require some crushing and grinding (e.g., Larsen et al., 2001). Pastoralists focus on very soft foods that impact tooth enamel minimally (e.g., Honeychurch, 2014). However, the partial eta squared effect size indicates that the effect of complexity on subsistence group membership is moderate (8.5%). So, while complexity is a factor, there is a good deal of variation in each group.
Foragers stand out again with regard to anisotropy, possessing significantly lower values than farmers and pastoralists. The low anisotropy found among foragers indicates that the jaw was moving in many directions during mastication. By contrast, farmers and pastoralists possess molars whose microwear suggests that the mandible moved in fairly constant directions during mastication. The effect size for anisotropy (0.047) is lower than that for complexity. This is not surprising since anisotropy failed to distinguish the farmers and pastoralists. But, the partial eta squared value and its CI also indicate the significant value generated by the NHST is not a Type I spurious result generated by large sample sizes, and it supports the statistically significant difference found here.
The results from H2 indicate that regardless of location (i.e., OW or NW) foragers generate microwear in similar ways, and farmers do the same. The data support the idea that foragers, as a whole, rely on a range of food items that are not often processed (or at least minimally so), whereas farmers as a whole tend to process their foods to a greater extent prior to consumption. Consequently, almost no hemispherical differences were found between NW and OW foragers and NW and OW farmers. In fact, the only difference that emerged was for anisotropy between the OW and NW foragers when the Natufians were included in the OW forager group. Removing them from the OW sample obviated the significant difference between the NW and OW foragers (see Table 5).
Not surprisingly for this test, the Hedges's g values are low and the Hedges's CIs include zero. When this happens, group membership has almost no effect on the dependent variable and supports the finding of a failure to reject the null hypothesis (Ferguson, 2009).
The significant difference in complexity between Early and Late farmers found in the H3 tests indicates farming diets in the OW got softer over time. This difference might simply reflect improved food processing techniques that introduced less grit into the diet. The Romans, for example, used large basalt rotary querns turned by draft animals rather than the earlier sedimentary stones used for manual grinding. Likewise, improvements in ceramics may have led to greater boiling (Barker, 1985). Either way, it looks like the diet softened from the Early Bronze to the Roman Age. Importantly, this conclusion is supported by the Hedges's g value, which is robust and its CI does not include zero. The persistence of the high anisotropy from the Early to Late groups is also interesting. It implies that masticatory movements in Early farmers remained nearly the same in the later groups, which indicates that farmers consumed foods that could be chewed with a more consistent jaw movement when compared to the foragers who had wear features going in more directions.
Microwear studies of nonhuman animals usually attribute high anisotropy values to the consumption of tough and/or fibrous foods, which require precise jaw movements during tooth–food–tooth occlusion (see Scott et al., 2012; Teaford & Ungar, 2014; Ungar, 2010, 2015). Herbivorous animals like bovids and certain nonhuman primates have high anisotropy values because of the grass-based diets they have (Schulz et al., 2013; Scott, 2012; Scott et al., 2012; Shearer et al., 2015). Herbivourous animals grind tough grasses with strong lateral mandibular movements that predominantly generate microwear striations (e.g., Solounias & Semprebon, 2002). Likewise, grass-eating gelada monkeys (Theropithecus gelada) have microwear dominated by scratches and high anisotropy (Scott et al., 2012).
Thus, it is difficult to give a precise explanation for the elevated farmer anisotropy values. While rare in the current study, foragers can have high anisotropy values (e.g., El Zaatari, 2008, 2010). Explanations for these instances of high forager anisotropy tend to connect diet to the ecogeographic region in which groups lived; in places where tough/fibrous foods were readily available sources of nutrition, they were consumed. El Zaatari and colleagues noted this relationship in recent archeological groups (El Zaatari, 2008, 2010), anatomically modern humans of the Upper Paleolithic (El Zaatari & Hublin, 2014), and Neandertals (e.g., El Zaatari, Grine, Ungar, & Hublin, 2011). Since then, other researchers have drawn similar conclusions, particularly regarding Neandertals (e.g., Karriger et al., 2016; Williams et al., 2018).
For farmers, however, the cause of their high anisotropy values is less clear even though it is a global phenomenon; farmers the world over tend to have high anisotropy. While ecogeographic explanations are likely part of the equation, farmers alter their environments to fit the needs of what they produce (Bellwood, 2005). One possibility is that the comparatively homogenous diets of farmers lead to persistent masticatory movements, which create microwear features following similar paths. But, at this point, explanations for farmer anisotropy remain elusive and require further investigation.
The K-means cluster analysis supports the overall findings from the ANOVA tests (Table 8). Where there is overlap between foragers and farmers, often the distinguishing factor is anisotropy. Foragers, for the most part, have low anisotropy values while farmers and pastoralists, tend to have higher anisotropies. In fact, the only foragers in this study to have high anisotropy were the Natufians. Farmers that engage in significant wild food exploitation, like the mixed economy populations of the late precontact in North America, are the most difficult for microwear to discern independently; thus mixed economy and forager groups end up in the same cluster.
9.1 Interpretations
This study has found that variation exists within and between these large subsistence groupings, populations, and localities with respect to the two DMTA variables considered (complexity, anisotropy). As such, some of the results question notions of simple forager-farmer-pastoralist typologies and boundaries. There are hard-food foragers and soft-food foragers as well as hard- and soft-food farmers. There are also high-anisotropy foragers and high-anisotropy farmers. In fact, it may be that in bioarchaeology the value of microwear is greatest when it provides an insight that was unexpected based upon a particularly site's subsistence record. While it is apparent that there are overarching trends in microwear signatures, namely that foragers tend to have high complexity and low anisotropy, while farmers tend to have low complexity and high anisotropy, the nuances and exceptions are equally valuable to discover. An example of this comes from the Natufians who clustered with the farmers via the cluster analysis (See Table 8). Recall that the Natufians are usually categorized as preagricultural foragers (Bar-Yosef, 1998). Their microwear signatures indicate that their preferred foods were very similar to what was later domesticated (Chiu et al., 2012; Schmidt et al., 2011). Thus, the microwear data support the notion that the Natufians do not neatly fall into either a forager or farmer category (see Bar-Yosef, 2002).
Low complexity foragers, such as those of the Archaic period in North America, are thought to be more reliant on meat since their stable isotope values indicate consumption of terrestrial animals (Schoeninger, DeNiro, & Tauber, 1983). High complexity foragers are usually people living in wooded areas who consume large quantities of nuts and practice a mixed economy. Low food processing is another contributor to very high complexity values among foragers, as is the case of Lagoa Santa, Brazil, whose groundstone assemblage lacks grinding instruments (Bueno & Isnardis, 2017). And, they have a low anisotropy value. In fact, anisotropy discriminates foragers and farmers as well as or better than does complexity (e.g., Schmidt et al., 2016).
Another interesting phenomenon that emerged from this study was the high complexity farmers. The aforementioned mixed economy people of North America had a subsistence record that helped to characterize their high complexities. But, the farmers from Kish, which is an early farming population from Iraq, has a mean complexity value similar to that of foragers. This may indicate the practice of a mixed economy or a type of agriculture not yet identified. Along these lines, the apparent dichotomization of the foragers and farmers in clusters 1 and 3 of the cluster analysis is accompanied by an overlap of foragers and farmers in cluster 2. This cluster appears to be a nexus of hard food consumption, since all of its members have elevated complexity values. Interestingly, there were no foragers with high complexity and high anisotropy. That condition was only found among the farmers. Finally, the pastoral people have microwear that is very similar to that of farmers, and pastoralism does not stand out as a separate cluster in the cluster analysis. Their very low complexity is somewhat unique, but their anisotropies are right in line with farmers. It is plausible that the pastoralist microwear signature is supporting the archeological record that indicates pastoralists consumed agricultural goods they acquired through trade.
10 CONCLUSION
The current study employed two DMTA variables—complexity and anisotropy—to distinguish between large samples of individuals representing foragers, farmers, and pastoralists from across the globe. Significant differences were found among all three subsistence groups. Based upon the effect indicators and their CI's, these statistically significant differences were not artifacts of the large sample sizes used in the study. Moreover, the results demonstrated subtleties within the farmers, particularly in complexity between early and late farmers. In general, foragers had higher complexity and lower anisotropy whereas farmers and pastoralists had lower complexity and higher anisotropy. Pastoralists had low complexities and high anisotropies that aligned them more with farmers than the foragers. Intrasubsistence group variation, however, points to important microwear nuances. For example, there were high complexity foragers and high complexity farmers. Indeed, the meaningfulness of DMTA rests in its ability to discern not only the large dietary differences, but also the subtle variation often gone undetected by other types of dietary reconstruction.
ACKNOWLEDGMENTS
Many thanks to the Editor, Associate Editor, and reviewers of this manuscript. Special thanks to Mark Teaford who provided extraordinary input. Others who helped with this project include Brenda Detty, Christa Kelly, Jessica Chevrolet, Molly Schmidt, and Gregory A. Reinhardt. This study, as a whole, benefited greatly from conversations with Jerry Rose and Peter Ungar. Additionally, we would like to thank: George Crothers of the William S. Webb Museum of Anthropology; Aleydis Van de Moortel, Eleni Zahou, and the Cobb Institute of Archeology; Philippe Mennecier and Fabrice Demeter (Musée de l'Homme). Schmidt's profilometry was funded by the National Science Foundation (BCS-0922930); Mahoney's Canterbury research was funded by the British Academy—Leverhulme Trust; Eng's research in Nepal was supported by funds awarded to M. Aldenderfer (NSF grant BCS-1528698, NGS grant 8810-10); Stojanowski's work was funded by Wenner-Gren Foundation for Anthropological Research (GR6698) and the National Science Foundation (BCS-0636066, BCS-0820805); Stone's work was funded by an LUROP Mulcahy Fellowship through Loyola University Chicago; da Gloria's research was funded by the São Paulo Research Foundation (process 2013/00069-0). Scott's work was funded by a Faculty of Arts Doctoral Award, University of Auckland; Willman was funded by the Leakey Foundation, Marie Skłodowska-Curie Actions (H2020-MSCA-IF-2016 No. 749188), AGAUR (Ref. 2017SGR1040) with URV (Ref. 2016PFR-URV-B2-17), and MINECO/FEDER (Ref. CGL2015-65387-C3-1-P).




