Determinants of terrestrial feeding in an arboreal primate: The case of the southern bamboo lemur (Hapalemur meridionalis)
Funding Information: This field research was result of generous financial and in-kind support provided by the American Society of Primatologists, Conservation International's Primate Action Fund, Idea Wild, Mohamed bin Zayed Species Conservation Fund (Project Number: 11253008), Primate Conservation Inc., and the Primate Society of Great Britain/Knowsley Safari Park.
Abstract
Objectives
The proximate and ultimate determinants that may have prompted some primates to shift from an arboreal to terrestrial feeding niche, whether due to environmental change, seasonality, and/or predation pressure, are poorly understood. Within a fragmented littoral forest in southeast Madagascar, an arboreal strepsirrhine population spends a large proportion of time on the ground, thus we aimed to identify which factors influence terrestrial feeding.
Methods
From January to December 2013, we conducted 103 full-day focal follows on three social groups of southern bamboo lemurs H. meridionalis. We continuously recorded feeding time on all arboreal and terrestrial items, as well as whether the focal individual was under the canopy or exposed, and the distance to their nearest conspecific neighbor. All observed food items were collected and analyzed for macronutrient content. Daily climatic variables (temperature, precipitation), resource seasonality, daily path length (DPL), along with dietary and predation risk proxies, were used as fixed effects in a linear mixed model, with the daily proportion of terrestrial feeding as the dependent variable.
Results
Our model indicated that daily terrestrial feeding increased at cooler temperature, was associated with reduced DPL, and the intake of dietary metabolizable energy increased as terrestrial feeding increased. All other fixed effects were not significant predictors.
Discussion
Our study provides a window into the ultimate determinants of niche expansion: ancestral primates, in absence of their primary resources, may have initially descended to the ground in peripheral population range areas where the benefits (e g., nutritional pay-off) out-weighed the costs.
1 INTRODUCTION
It has been suggested that ancestral eutherian orders, including placental mammals, are likely characterized by a terrestrial evolutionary history, with subsequent transitions to arboreality occurring multiple times to fulfill various ecological niches (reviewed in Ji et al., 2002; Szalay, 2007). In contrast, arboreality is the primitive condition for the Order Primates, having initially evolved in Euarchonta, that is, ancestral mammals from which Primates radiated (Bloch & Boyer, 2002; Kirk, Lemelin, Hamrick, Boyer, & Bloch, 2008; Sussman, 1991; Szalay, 2007). The subsequent evolutionary shift in some primate species from an arboreal to terrestrial niche is shown through various morphological adaptations, for example, limb, dental, postcranial, etc. (Fleagle, 2013; Gebo, 1996; Gebo & Sargis, 1994; Motsch et al., 2015). The evolutionary pressures that led to a terrestrial niche, however, are poorly supported by empirical data.
Foraging is often considered to be a predator-sensitive behavior, whereby foraging success may be outweighed by the necessity to minimize the risk of predation (Altmann, 1974b; Miller, 2002a; Schoener, 1971). For example, a desert population of baboons (Papio ursinus) was shown to exploit low-risk, low-quality foraging sites rather than risk foraging on high-quality foods where the potential of predation was higher (Cowlishaw, 1997). Similarly, the impact of potential predator risk on primate foraging behavior has been repeatedly shown (Cords, 2002; Miller, 2002b; Overdorff, Strait, & Seltzer, 2002; Sauther, 2002). While some argue that there is a higher risk of predation on the ground (Janson & Goldsmith, 1995; van Schaik, 1983; Wrangham, Gittleman, & Chapman, 1993), others have suggested that primates with arboreal or terrestrial lifestyles may be equally susceptible to predators (Cheney & Wrangham, 1987; Hart, 2007; Isbell, 1994; Janson & Goldsmith, 1995), depending on whether their feeding or resting sites are more exposed (Janson, 1998; van Schaik & van Noordwijk, 1989). It has also been suggested that predation risk should be greater for animals whose nearest conspecific neighbors are farther away (Hamilton, 1971; Phillips, 1995; Treves, 1998). Thus, if individuals on exposed substrates are at greater risk of predation, then the presence of neighbors may provide some protection (Di Fiore, 2002).
In addition to predation pressure, food distribution and dietary quality are also considered to have an influence on whether primate species live arboreally or terrestrially (Cant, 1992; Campbell et al., 2005; Janson, 1990; Jolly, 1985; Xiang, Huo, Xiao, Quan, & Grueter, 2009). Arboreal primates face the risk of descending to the ground primarily to gain access to water or to obtain certain amino acids and/or minerals (Campbell et al., 2005; Izawa, 1993; Link, Galvis, Fleming, & Di Fiore, 2011). This is quite different from a dietary niche expansion, whereby animals may be seasonally supplementing their daily nutritional intake during a lean season (Barnett et al., 2012; Grueter et al., 2009). Thus, the nutritional gain from ubiquitous (i.e., rather than clumped) terrestrial food items during periods of food scarcity in the arboreal stratum may have been a catalyst in the transition of an arboreal mammal to a terrestrial dietary niche, although other factors are likely to have played a role, for example, potential predation risk, thermoregulation, and/or energetic costs of locomotion.
Among the strepsirrhine primates of Madagascar, the ring-tailed lemur (Lemur catta) is the most terrestrial species, spending approximately 30 to 40% of its time on the ground (Cameron & Gould, 2013; Jolly, 1966; Sauther, Sussman, & Gould, 1999; Sussman, 1974). Although duration tends to be minimal, the occasional occurrence of terrestrial traveling and/or foraging is exhibited among other lemurs, for example, collared brown lemurs (Eulemur collaris; Lazdane, Broll, Theisinger, Bearder, & Donati, 2014), crowned lemurs (E. coronatus; Wilson, Stewart, Ramangason, Denning, & Hutchings, 1989), red-fronted lemurs (E. rufifrons; Overdorff et al., 2002; Sussman, 1974), diademed sifaka (Propithecus diadema; Irwin et al., 2007), Milne-Edward's sifaka (P. edwardsi; Overdorff et al., 2002), Verreaux's sifaka (P. verreauxi; Brockman, Godfrey, Dollar, & Ratsirarson, 2008; Richard, 1974), and indri (Indri indri; Pollock, 1975). Bamboo lemurs (Hapalemur spp. and Prolemur simus) are no exception, having been observed to feed on the ground (Eppley & Donati, 2009; Eppley, Verjans, & Donati, 2011; Grassi, 2006; Overdorff, Strait, & Telo, 1997; Tan, 1999; Wright, 1986). However, bamboo lemurs are mostly arboreal, typically exploiting the low to mid-canopy habitat niche while relying on their cryptic behavior as an anti-predator strategy (Tan, 2006). Like their name indicates, they are known for their dietary specialization on bamboo, a subfamily of grasses that is widespread throughout the eastern forests of Madagascar (Dransfield, 2000), and at times can make up 85–95% of the bamboo lemur's diet at certain study sites (Grassi, 2006; Overdorff et al., 1997; Tan, 1999; Wright, 1986). The Alaotran gentle lemur has been an unusual exception within the genus, with its entire population living in the wetlands around Lac Alaotra, a habitat without bamboo. Here, Hapalemur alaotrensis have a diet that contains reeds and sedges, yet their dietary breadth remains low (∼11spp.) possibly due to the paucity of food options (Mutschler, 1999). Similarly, at the site of Mandena in southeast Madagascar, southern bamboo lemurs (Hapalemur meridionalis) inhabit an area that is also devoid of bamboo (Eppley et al., 2015a; Rabenantoandro, Randriatafika, & Lowry, 2007). Lacking the primary food resource for the genus, H. meridionalis focus a portion of their diet on various terrestrial grasses and spend nearly 70% of their feeding time on the ground during the austral winter, an exceedingly large amount of time compared with congeners (Eppley & Donati, 2009; Eppley et al., 2011). Their terrestrial grazing often takes place in a sparsely canopied swamp/marsh habitat (Eppley & Donati, 2009; Eppley et al., 2015a), potentially increasing their susceptibility to both aerial and terrestrial predation (Karpanty, 2006; Karpanty & Wright, 2007).
- Terrestrial feeding would be seasonal, specifically increasing during the cool, dry austral winter when dietary resources (e.g., ripe fruits, flowers, and flushing leaves) become more scarce (Bollen & Donati, 2005; Campera et al., 2014).
- As such, we further predicted that the daily nutritional intake of terrestrial food items would represent a higher dietary quality (i.e., protein/fiber ratio and metabolizable energy) than foods in the arboreal strata at that time, thus representing a benefit for their increased utilization of a potentially risky stratum.
- As daily path length (DPL) is a response to variation in resource distribution (Koenig, Borries, Chalise, & Winkler, 1997; Raño, Kowalewski, Cerezo, & Garber, 2016), shorter DPL will predict increased terrestrial feeding due to the ubiquity of terrestrial food items throughout the landscape.
- Furthermore, we predicted that the perceived risk of predation would be greater when bamboo lemurs fed terrestrially (compared to arboreal feeding), and thus individuals should maintain closer proximity to group members when feeding on the ground.
2 METHODS
2.1 Study site
Our study was conducted in the protected area of Mandena (24°95′S 46°99′E) in the extreme southeast of Madagascar, approximately 10 km north of Fort-Dauphin. This 230 ha area consists of fragmented and degraded littoral forest and interspersed, seasonally-inundated swamp (Eppley et al., 2015a). Among the most threatened habitats in Madagascar (Bollen & Donati, 2006; Ganzhorn, Lowry, Schatz, & Sommer, 2001), littoral forests occur within 3 km of the coast and are characterized as having a relatively low canopy that grows on sandy substrates (Consiglio et al., 2006; Dumetz, 1999). The vertical structure of the Mandena littoral forest has an average forest canopy height of approximately 7 m while the surrounding swamps maintain a slightly lower average canopy height of 6.5 m (Eppley et al., 2015a).
To assess daily climatic factors, which can be highly variable within the littoral zone, temperature (°C) was recorded in 30-min intervals using four Lascar EL-USB-1 data loggers (Lascar Electronics, Inc.; Erie, PA), operated by custom software (EasyLog USB Version 5.45, Lascar Electronics, Inc.). These were located in each of the Mandena habitats to provide daily averages. Precipitation (mm) was measured daily at 6:00 h using a rain gauge placed within the study site.
2.2 Study species
Southern bamboo lemurs (Hapalemur meridionalis) are relatively small-bodied primates (1.072 ± 0.107 kg; N = 15) that exhibit a cathemeral activity pattern (Eppley, Ganzhorn, & Donati, 2015b; Eppley, Hall, Donati, & Ganzhorn 2015c; Fausser, Prosper, Donati, Ramanamanjato, & Rumpler, 2002). They live in small social groups, typically one or two adult females and one or two adult males, with an average size of 5.6 ± 1.5 individuals (N = 5 groups) (Eppley, Ganzhorn, & Donati, 2016b). Similar to congeners, they are classified as folivores (Eppley et al., 2011).
To expedite our ability to locate these highly cryptic animals, we captured and collared ten adult H. meridionalis across four neighboring social groups between October and December 2012. Details of the capture protocol followed have been previously described in Eppley et al. (2015b).
2.3 Resource seasonality data
To estimate monthly variation in food availability, phenology data were recorded for plant species (N = 100) known to produce foods consumed by lemur species within Mandena. Utilizing an established transect that includes both littoral forest and swamp habitats, the first five to six mature (DBH ≥ 10 cm) individuals encountered for each plant species were selected from which to collect phenological data. Plants (N = 517) were observed twice a month for the presence/absence of flowers and fruits (Bollen & Donati, 2005). While we did not collect phenology data on young leaves and grass availability, the former has been previously shown to be highly correlated with fruit availability in the littoral forest habitat (Bollen & Donati, 2005).
2.4 Behavioral data
From January to December 2013, we conducted full-day focal follows (from sunrise to sunset) for approximately five days a month with Groups 1, 2, and 4 each, while Group 3 was used exclusively for home range data collection. Identification of individuals was made using radio-tracking tags with colored pendants, with all adult individuals (N = 10) from our three focal groups sampled at least once each month. Continuous sampling (Altmann, 1974a) was utilized each time the focal was observed feeding. This included the exact time spent feeding (timed to the second) per food item(s) while noting the plant species. Height was recorded as meters above ground for each feeding bout. As individuals occasionally move between strata while feeding, we time-stamped changes (to the second) in height so as to be exact in how much time they spent feeding on an item in each stratum. A new bout was recorded if there was a 60-sec interval with no feeding. Furthermore, to estimate DPL, GPS coordinates were recorded in UTM every 15 min.
To estimate exposure to diurnal birds of prey, we collected instantaneous point sampling (Altmann, 1974a) at 5-min intervals of whether the focal subject was located directly under canopy cover, or if the individual was exposed (i.e., no canopy directly above the focal). Two species of hawk are present in Mandena, Madagascar harrier-hawk Polyboroides radiatus and Henst's goshawk Accipiter henstii (TME, personal observation), both of which represent a potential threat for adult bamboo lemurs (Karpanty, 2006; Karpanty & Wright, 2007). A third large aerial raptor, Madagascar buzzard Buteo brachypterus, is also present in Mandena and has been observed to prey on medium-sized lemurs (Wright, Heckscher, & Dunham, 1998). Given the various hunting strategies of these raptors (Brockman, 2003) and the habitat differences, our method may not provide an accurate measure of predation risk. However, playback experiments of aerial predators have shown Hapalemur to descend in the canopy in response to raptor calls (Karpanty & Wright, 2007). As our main goal was a comparative measure between feeding strata (i.e., arboreal vs. terrestrial), we considered our canopy exposure method as an acceptable proxy.
Predation risk of Hapalemur spp. is not limited to aerial predators; Eupleridae carnivores, for example, fossa Cryptoprocta ferox (Goodman & Pidgeon, 1999; Sterling & McFadden, 2000), as well as large snakes, for example, Madagascar tree boa Sanzinia madagascariensis (formerly Boa manditra; Goodman, O'Conner, & Langrand, 1993; Rakotondravony, Goodman, & Soarimalala, 1998) and Dumeril's boa Acrantophis dumerili (Eppley & Ravelomanantsoa, 2015), present potential arboreal and terrestrial predatory threats, respectively. Although bamboo lemurs are known for their cryptic nature, other evolutionary anti-predator strategies may include group defense, dilution of risk, or increased vigilance (Hamilton, 1971; Janson, 1992). To test whether H. meridionalis used these strategies, we instantaneously recorded the nearest neighbor to the focal every 5 min, categorized as close (≤ 3 m) and far (> 3 m). This allowed us to calculate daily proportions for having a close neighbor for both arboreal and terrestrial feeding.
2.5 Nutritional analyses
We collected samples from all known food items we observed the lemurs to consume. These included grasses, piths, young and mature liana leaves, young liana stems, flowers, unripe and ripe fruits, fungi, and soil, collected directly from feeding trees and/or grazing sites on the same day or at the same time the following day. Samples were weighed with an electronic balance (fresh weight), dried overnight at approximately 40°C in a commercial electric drying oven in an office with stable electricity supply, and weighed again (dry weight) at the field site. Dry matter specimens were exported to the University of Hamburg and biochemical analyses on all food items were conducted in 2013–2014. Specimens were then ground to pass a 1 mm sieve and dried again at 50–60°C before analyses. Nitrogen was measured via the Kjeldahl method while soluble proteins were assessed via BioRad after extraction of the plant material with 0.1 N NaOH for 15 h at room temperature. Soluble carbohydrates (SC) were extracted with 50% methanol. Concentrations of soluble sugars were determined as the equivalent of galactose after hydrolization of 50% methanol extract. Specimens were analyzed for neutral (NDF) and acid (ADF) detergent fibers, with NDF representing all the insoluble fiber (cellulose, hemicellulose and lignin) and ADF representing the fiber fraction containing cellulose and lignin. Lipid content was determined by extraction using petroleum ether, followed by evaporation of the solvent. Detailed reviews of the procedures and their biological relevance are provided by Ortmann, Bradley, Stolter, and Ganzhorn (2006), Donati, Bollen, Borgognini-Tarli, and Ganzhorn (2007), and Rothman, Chapman, and Van Soest (2012).
2.6 Data analyses
Our examination of the southern bamboo lemur feeding ecology sought to assess dietary diversity for annual diets in each of the three social groups via species numbers and the Simpson's diversity index (Begon, Harper, & Townsend, 1996).
For resource seasonality, we calculated a monthly proportion of flower/fruit presence, allowing us to generate a dichotomous variable (abundant/lean) for each month. Similar to previous studies (Bollen & Donati, 2005; Campera et al., 2014), Mandena resource abundance corresponded to October–March, whereas resource scarcity corresponded to April–September.
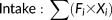
where Fi is the daily proportion of feeding records and Xi is the percentage of dry matter of each chemical parameter for the ith item. Our first measure of dietary quality, protein-to-fiber ratio, was calculated as crude protein/acid detergent fiber (Milton 1979; Mutschler 1999), using a conversion factor of 6.25 to estimate crude protein from the total nitrogen present via the Kjeldahl method (Ortmann et al., 2006). This ratio is a useful indicator of whether certain species choose to consume a particular leaf species (Davies, Bennett, & Waterman, 1988; Ganzhorn 1992; Milton 1979, 1998; Simmen, Tamaud, & Hladik, 2012); however, it may only explain leaf choice for some groups but not others (Chapman & Chapman, 2002; Chapman, Chapman, Naughton-Treves, Lawes, & McDowell, 2004) and its biological meaning has been questioned based on theoretical (Wallis et al., 2012) and empirical grounds (Gogarten et al., 2012). Nevertheless, we use this ratio as one component in our analyses to allow comparisons with previous studies.
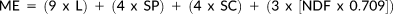
For the sake of this study, we are treating feeding height as a simple dichotomy, that is, arboreal (> 0 m) or terrestrial (0 m). To determine the differences in nutritional gain between the arboreal and terrestrial niche, we calculated the two aforementioned nutritional quality measures based on food item intake time within each strata. Furthermore, we calculated a daily proportion of canopy exposure for both strata.
DPL (m) was calculated as the sum of accumulated straight-line distances between successive GPS coordinates from daily full-day focal follows (Suarez, 2006).
To determine which factors influenced terrestrial feeding, we fitted linear mixed-effects models (LMM) in R statistical software (R Development Core Team, 2014) using the lmer function of the lme4 package (Bates, Maechler, & Bolker, 2012), with the daily proportion of time spent feeding terrestrially as a dependent response variable. We only included data from days in which the focal subject was observed for ≥80% of the day length, as determined by sunrise and sunset. To reduce the necessity for running multiple LMMs and increase statistical power, we calculated the daily differences from terrestrial and arboreal proportional values for the following fixed effects: weighted average of protein-to-fiber ratio and weighted average of metabolizable energy (both as proxies for dietary quality), canopy exposure (as a proxy of exposure to birds of prey), as well as distance to nearest neighbor (proxy for perceived predation risk). This provided one overall comparative value per day rather than one per strata. In addition, we included DPL of the focal individual, resource seasonality, as well as climatic variables of daily mean temperature and daily total precipitation as fixed effects. Groups were included as random effect to control for repeated sampling. We then used the anova function to calculate likelihood ratio tests for model comparison, allowing us to determine which model had the best explanatory power by comparing Akaike's Information Criterion (AIC) values for all possible models. p values were obtained with a likelihood ratio test using the afex package (Singmann, 2014), developed for R statistical software (R Development Core Team, 2014) with significance considered at p < .05. Residuals from the analysis were normally distributed according to the Kolmogorov–Smirnov test.
3 RESULTS
3.1 Dietary diversity
We observed H. meridionalis for 1,762 h, resulting in 694 h of feeding recorded. Overall, southern bamboo lemurs consumed 86 different food items from 72 distinct species in Mandena, with the top ten species contributing 75.95% of their total feeding record. These lemurs appear to rely heavily on a few key liana and graminoid species for the majority of their daily food intake (Table 1). Graminoids (i.e., species of the families Poaceae and Cyperaceae) are almost exclusively eaten from a terrestrial position, and occur throughout the Mandena littoral forest and marsh/swamp. Concerning dietary diversity, Groups 1 and 4 consumed 56 distinct species while Group 2 had a slightly lower diversity with 47 species consumed during the year (Table 1). Group 1 had both the highest plant species and family diversity of consumed foods. Terrestrial feeding was not limited to just graminoid species (and soil and water), but rather comprised 29 different items that included forbs, fungi, young liana stems, and fallen fruit. The largest median proportion of time spent feeding terrestrially was in June (0.85), with the greatest number of food items consumed terrestrially occurring in August (N = 25). While both of these large values occurred during the austral winter, terrestrial feeding by bamboo lemurs exhibited substantial variation across the entire study period (Figure 1). This was perhaps shown best in the month of February, which was also the month with the highest precipitation, as it exhibited both the lowest median proportion of terrestrial feeding (0.01), as well as the fewest number of food items consumed while on the ground (N = 5).
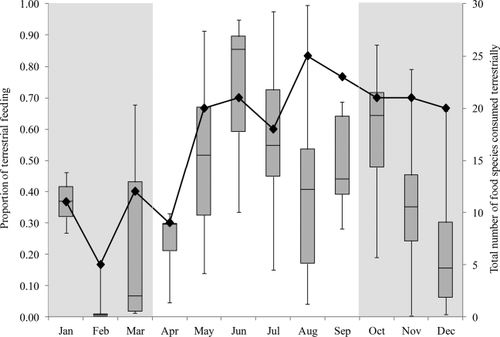
Monthly proportion of terrestrial feeding (box plots indicate medians, interquartiles and ranges) and monthly total of food species consumed terrestrially (line with diamond markers) by H. meridionalis in Mandena between January and December, 2013. Period of food resource abundance denoted with gray background, while lean period denoted with white background
| Group 1 | Group 2 | Group 4 | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Food category | No. species | TFR (%) | No. species | TFR (%) | No. species | TFR (%) | No. species | TFR (%) |
| Grass | 8 | 25.44 | 7 | 35.64 | 7 | 44.29 | 8 | 34.34 |
| Pith | 11 | 18.89 | 8 | 5.22 | 14 | 15.02 | 14 | 12.75 |
| Liana stem | 5 | 15.65 | 5 | 12.27 | 4 | 6.35 | 5 | 11.17 |
| Liana leaves | 5 | 10.99 | 5 | 11.32 | 7 | 4.54 | 7 | 8.75 |
| Fruit | 23 | 19.25 | 19 | 27.98 | 18 | 9.78 | 34 | 18.58 |
| Flower | 9 | 7.25 | 7 | 11.18 | 7 | 20.78 | 10 | 12.78 |
| Fungi | 4 | 2.32 | 2 | 0.98 | 3 | 0.72 | 4 | 1.31 |
| Soil | Y | 0.09 | Y | 0.20 | Y | 0.02 | Y | 0.10 |
| Water | Y | 0.11 | Y | 0.49 | Y | 0.04 | Y | 0.21 |
| Total | ||||||||
| Species consumed | 56 | 47 | 56 | 72 | ||||
| ≥ 1% | 15 | 14 | 20 | 18 | ||||
| Simpson's D | 11.97 | 6.61 | 7.79 | 12.08 | ||||
| Family consumed | 29 | 27 | 33 | 37 | ||||
| ≥ 1% | 13 | 10 | 16 | 14 | ||||
| Simpson's D | 6.91 | 5.49 | 4.49 | 6.26 | ||||
- TFR = total feeding record.
- Note. Soil and water were excluded from diversity indices.
In terms of resource seasonality, the average daily percentage of terrestrial feeding by H. meridionalis was 30.91 ± 4.08% (±SE) during resource abundant months, compared to 52.74 ± 3.24% (±SE) daily feeding on the ground during months of resource scarcity. Furthermore, while southern bamboo lemurs exhibited an average DPL of 903.91 ± 373.05 m, they displayed some monthly variation, with the longest DPL equaling 2,224.34 m in February and the shortest DPL equaling 277.15 m in May (Figure 2).
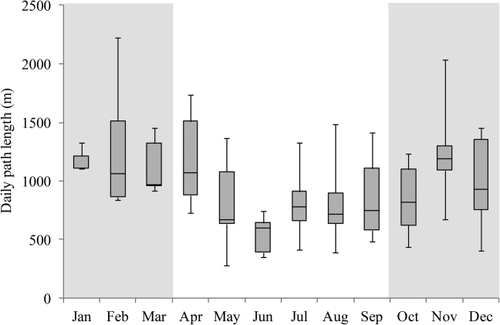
Box plots of the medians, interquartiles, and ranges of daily path length traveled monthly by H. meridionalis in Mandena between January and December, 2013. Period of food resource abundance denoted with gray background while lean period denoted with white background
When analyzed for macronutrient compositions, terrestrial food items were shown to have high nitrogen and fiber (NDF) content compared to those items consumed arboreally, while arboreal food items had higher SC, ADF, and phenols (Table 2). During our full-day focal follows (N = 103), terrestrial feeding time of these lemurs averaged 143.20 ± 106.71 min (±SD) and arboreal feeding time averaged 158.08 ± 85.69 min (±SD), daily. These lemurs also exhibited differences between feeding strata in both dietary quality, that is, terrestrial food items represented higher PF ratio and ME (kcal g−1) compared to arboreal food items, and predation proxies, that is, focal lemurs were more often exposed with no canopy above them when feeding terrestrially, yet they maintained closer proximity to their nearest neighbor in this stratum compared to when they fed arboreally (Table 3).
| Category | N | SP | SC | NDF | ADF | Lipid | Tannins | Phenol | Ash |
|---|---|---|---|---|---|---|---|---|---|
| Grass | 1.79 | 2.06 | 4.10 | 67.34 | 31.76 | 1.48 | 0.00 | 0.79 | 7.59 |
| N = 10 | 1.67–2.16 | 1.83–2.68 | 3.47–4.39 | 62.88–70.62 | 28.32–35.00 | 1.08–1.81 | 0.00–0.00 | 0.59–0.92 | 6.75–8.44 |
| Pith | 1.25 | 2.28 | 3.37 | 62.33 | 37.87 | 1.11 | 0.27 | 0.60 | 10.27 |
| N = 13 | 1.05–1.55 | 2.07–3.72 | 2.65–3.91 | 59.23–66.89 | 35.98–46.04 | 0.78–1.57 | 0.00–0.42 | 0.54–0.85 | 7.81–11.14 |
| Liana stem | 1.36 | 1.50 | 3.50 | 52.41 | 36.06 | 1.37 | 0.00 | 0.48 | 8.21 |
| N = 7 | 1.23–2.83 | 1.23–2.38 | 3.18–9.90 | 44.64–56.36 | 28.40–41.25 | 1.22–1.60 | 0.00–0.00 | 0.36–0.86 | 5.96–9.31 |
| YL | 1.82 | 1.82 | 4.25 | 37.87 | 26.92 | 1.86 | 0.00 | 0.91 | 9.38 |
| N = 6 | 1.52–1.98 | 1.48–2.50 | 4.07–7.46 | 30.55–57.52 | 21.02–34.57 | 1.18–2.74 | 0.00–0.00 | 0.51–1.74 | 7.40–11.75 |
| ML | 1.49 | 1.06 | 4.52 | 45.66 | 31.75 | 4.65 | 0.23 | 0.49 | 11.05 |
| N = 1 | |||||||||
| U. Fruit | 0.78 | 4.71 | 1.94 | 65.12 | 48.07 | 3.77 | 0.22 | 0.57 | 7.00 |
| N = 3 | 0.76–0.98 | 3.34–4.82 | 1.34–2.71 | 59.72–68.77 | 43.19–52.31 | 2.14–5.21 | 0.11–0.26 | 0.47–0.69 | 4.89–7.42 |
| R. Fruit | 0.69 | 2.35 | 6.83 | 56.79 | 40.50 | 3.35 | 0.00 | 0.85 | 3.67 |
| N = 33 | 0.59–0.87 | 1.94–4.07 | 4.41–10.67 | 45.14–61.21 | 33.22–44.50 | 1.48–4.47 | 0.00–0.49 | 0.65–1.37 | 2.90–4.50 |
| Flower | 0.86 | 4.33 | 7.39 | 36.97 | 29.80 | 1.55 | 0.29 | 1.90 | 6.27 |
| N = 11 | 0.74–1.00 | 3.14–6.14 | 5.81–10.06 | 32.10–53.45 | 25.22–35.48 | 1.09–1.81 | 0.00–0.90 | 1.19–4.08 | 5.59–8.48 |
| Fungi | a | 1.87 | 3.70 | 59.98 | 23.95 | 0.60 | 0.00 | 0.24 | 2.74 |
| N = 4 | 1.43–3.17 | 3.41–5.45 | 55.33–64.60 | 19.97–33.66 | 0.43–0.82 | 0.00–0.00 | 0.16–0.35 | 2.15–3.33 | |
| Soil | 0.13 | 0.24 | 0.14 | 97.84 | 97.14 | 0.09 | 0.00 | 0.02 | 95.28 |
| N = 1 | |||||||||
| Arboreal | 0.86 | 2.28 | 5.65 | 54.31 | 37.24 | 1.82 | 0.00 | 0.77 | 4.62 |
| N = 71 | 0.65–1.24 | 1.66–4.07 | 3.50–9.65 | 44.84–62.33 | 29.80–44.50 | 1.12–3.99 | 0.00–0.27 | 0.54–1.37 | 3.26–7.45 |
| Terrestrial | 1.64 | 2.28 | 3.91 | 64.44 | 32.14 | 1.37 | 0.00 | 0.76 | 8.01 |
| N = 27 | 1.21–2.00 | 1.81–2.94 | 3.19–4.90 | 59.04–69.02 | 27.56–36.62 | 0.73–1.66 | 0.00–0.24 | 0.54–0.94 | 6.75–10.62 |
- N = nitrogen; SP = soluble protein; SC = soluble carbohydrates; NDF = neutral detergent fiber; ADF = acid detergent fiber.
- As some food items were consumed in both strata, those values were used in both. Values are medians and quartiles.
- a Nitrogen content for the category Fungi is not reported as their cell walls contain a large amount of non-protein nitrogen (chitin), thus introducing potential error.
| Dietary proxy | Predation proxy | ||||
|---|---|---|---|---|---|
| Feeding (%) | PF ratio | ME (kcal g−1) | Canopy exposure (%) | NN (%) | |
| Arboreal | |||||
| Median | 57.26 | 0.19 | 1.68 | 4.59 | 46.43 |
| Quartiles | 32.56–76.24 | 0.15–0.23 | 1.56–1.78 | 0.63–13.63 | 31.73–60.17 |
| N | 103 | 103 | 103 | 103 | 103 |
| Terrestrial | |||||
| Median | 42.74 | 0.35 | 1.87 | 6.71 | 66.67 |
| Quartiles | 23.76–67.44 | 0.31–0.38 | 1.84–1.89 | 0.00–18.44 | 45.33–83.03 |
| N | 103 | 103 | 103 | 103 | 103 |
- PF ratio = Protein-to-Fiber ratio; ME = Metabolizable Energy; NN = Nearest Neighbor.
- All values were calculated as daily proportions from full-day focal follows in Mandena from January to December, 2013.
3.2 Proximate and ultimate determinants of terrestrial feeding
To determine which factors best predicted a greater daily proportion of terrestrial feeding, we used a LMM. The best-fit model included significant values for nutritional proxies (metabolizable energy alone and as an interaction with protein-to-fiber ratio), DPL, and the climatic influence of temperature (AIC = −7.604, χ2 = 11.435, df = 1, p < .001; Table 4). In particular, food items consumed on the ground contained more metabolizable energy (ME), which was positively related to terrestrial feeding time. Furthermore, DPL was shorter on days with increased terrestrial feeding, resulting in longer DPLs when bamboo lemurs spent more time arboreally feeding. Additionally, daily terrestrial feeding increased on days with low temperatures. The only interaction that was included in the best-fit model, PF × ME, indicated that for every 0.1 kcal g−1 that terrestrial ME is greater than arboreal ME when there was no difference in PF, H. meridionalis spent 2.3–5.6% more time feeding on the ground that day. Thus, in terms of the significant interaction of PF × ME, for every percent dry matter increase in the difference of PF ratio, the slope of ME increased by 2.04 kcal g−1. Resource seasonality, daily precipitation, along with the focal individual's proximity to nearest neighbor, canopy exposure, and protein–fiber ratio (PF) intake were not significant predictors of daily increases in terrestrial feeding.
| Variable | β | SE | 95% CI | t | p |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Intercepta | 1.91 | 0.46 | 1.04, 2.78 | 4.13 | |
| Canopy Exposure | −0.06 | 0.10 | −0.24, 0.12 | −0.61 | .52 |
| Nearest Neighbor | −0.02 | 0.08 | −0.16, 0.13 | −0.19 | .84 |
| Protein–Fiber Ratio (PF) | −0.05 | 0.27 | −0.56, 0.46 | −0.18 | .85 |
| Metabolizable Energy (ME) | 0.40 | 0.09 | 0.23, 0.56 | 4.50 | <.0001 |
| Daily Path Length | −0.31 | 0.13 | −0.56, −0.06 | −2.30 | .02 |
| Season | 0.04 | 0.07 | −0.10, 0.18 | 0.56 | .56 |
| Temperature | −0.03 | 0.01 | −0.05, −0.01 | −2.72 | <.01 |
| Precipitation | −0.01 | 0.00 | −0.01, 0.00 | −1.47 | .12 |
| PF × ME | 2.09 | 0.63 | 0.90, 3.28 | 3.30 | <.001 |
| Random effect | |||||
| Group | Variance | 0.00 | |||
| Residual | Variance | 0.22 | |||
- N = 103 days.
- p values (significant at p < .05, indicated in bold) were obtained using likelihood-ratio test
- SE = standard error; CI = confidence interval.
- a Note. The intercept is the predicted proportion of terrestrial feeding when all predictors are zero. Given that zero is outside the data range for multiple predictors of the model, the intercept itself does not provide a meaningful proportion itself.
4 DISCUSSION
Our model indicated that southern bamboo lemurs in Mandena increased their daily terrestrial feeding in cooler temperatures, with shorter DPL, and dietary metabolizable energy increased from feeding on the ground. All other fixed effects were not significant predictors of terrestrial feeding.
While fallen fruits were occasionally fed on from a terrestrial position, the majority of the southern bamboo lemur diet in this stratum consisted of non-bamboo grasses and Cyperaceae pith. In fact, southern bamboo lemurs were not observed to feed on any tree species' leaves; rather, they were only seen consuming the leaves of grasses, liana leaves, and other terrestrial ground cover (such as Asiatic pennywort Centella asiatica, Apiaceae). Within each group, young liana stems and their leaves (mostly from Baroniella camptocarpoides and Secamone sp.) constituted a large portion of their diet. These young lianas were only distributed within the littoral forest, however, while heavily consumed terrestrial grasses such as Panicum parvifolium and Stenotaphrum dimidiatum were distributed across both the littoral forest and the swamps. The ubiquity of grasses and reeds in Mandena would appear to provide folivorous primates with an excellent opportunity to expand their dietary niche within this fragmented and degraded littoral landscape.
The southern bamboo lemurs of Mandena display a dietary breadth beyond what has been previously recorded for any Hapalemur spp. (Table 5). Furthermore, their ability to include such a wide variety of fruits (34 spp.) in their dietary niche is exceptional for a folivorous species, which in fact was more than the total number of food species consumed by all other congeners. This was not entirely unexpected as H. griseus have been recorded to eat multiple fruit species in Ranomafana (Grassi, 2001; Tan, 1999), but these are proportionally limited in comparison. Southern bamboo lemurs showed substantial peaks in fruit consumption in February, July/August, and December, the latter two periods being almost solely based on Uapaca spp. fruiting (Eppley, unpublished data). The low frequency of terrestrially consumed food species observed in February (Figure 1) is potentially due to the increased rainfall during that month, which increased water depth in the swamp areas by approximately two meters and inhibited our ability to follow the animals there (Eppley et al., 2015a), thus biasing our full-day focal observations to days spent in relatively drier areas. Whether or not conspecifics of H. meridionalis in larger continuous forests consume a more specialized diet, these data suggest that based on this fragmented population the species should be considered feeding generalists.
| Congener | Species | Families | Site | Reference |
|---|---|---|---|---|
| H. alaotrensis | 11 | 8 | Lac Alaotra | Mutschler, 1999 |
| H. aureus | ≥21 | 15 | Ranomafana | Tan, 1999 |
| H. griseus | ≥24 | 12 | Ranomafana | Tan, 1999a |
| 22–31 | na | Ranomafana | Grassi, 2001 | |
| 12 | 8 | Andasibe | Overdorff et al., 1997 | |
| H. meridionalis | 72 | 37 | Mandena | This study |
| P. simus | 7 | 3 | Ranomafana | Tan, 1999 |
- na = information not available.
- a H. griseus noted as having been observed feeding on ≥ 40 food species (Tan, 2006).
Gelada baboons (Theropithecus gelada) are often regarded as the only graminivorous (i.e., grass-eating) primate and as such, an excellent model for early primates in savannah-type ecosystems (Dunbar, 1983; Dunbar & Bose, 1991; Fashing, Nguyen, Venkataraman, & Kerby, 2014). As previous studies and our results showed, H. meridionalis exploit a similar niche in which they focus their dietary efforts on graminoids (Eppley & Donati, 2009; Eppley et al., 2011, 2015c). Gelada baboons are large-bodied, large social group monkeys that inhabit high-altitude grasslands with practically no forest cover whereas bamboo lemurs are considerably smaller-bodied, family unit-living primates. While no extant predators (other than humans) remain in the environments where geladas live (Gippoliti & Hunter, 2008), various predators exist for bamboo lemurs; therefore, the risks imposed on these two species for terrestrial feeding are wholly disparate. Thus, it would appear that the bamboo lemurs in Mandena provide a suitable model with which to examine the benefits of terrestriality in a forest environment, adding complexity to the evolutionary scenarios of primate terrestriality.
4.1 Resource seasonality
Our data show that the daily proportion of terrestrial feeding increased when the temperature decreased. Although these colder temperatures were often within the austral winter, the corresponding resource seasonality was shown to not significantly predict increased terrestrial feeding. This contrasts with black-and-white snub-nosed monkeys (Rhinopithecus bieti) which have been observed to seasonally descend to the ground to feed on terrestrial grasses and bamboo shoots (Ding & Zhao, 2004; Xiang, Huo, Xiao, Quan, & Grueter, 2007), possibly representing additional dietary quality during the nutritionally lean season (Grueter et al., 2009; Xiang et al., 2009). Feeding on the ground for increased nutrition has also been suggested for the semi-terrestrial Semnopithecus sp. (Newton, 1992). Unlike these other primates, terrestrial feeding by H. meridionalis occurred year-round and was not seasonally determined by resource lean periods. More specifically, the expansion to terrestrial graminoids is not due to the seasonal lack of available fruits and flowers.
4.2 Nutritional pay-off
As we predicted, the nutritional quality of bamboo lemur daily intake increased with terrestrial feeding, an interesting finding considering that the foods available on the ground are mostly graminoids, which are typically assumed to be of low nutritional quality with tough and abrasive properties (Jablonski, 1994; Venkataraman et al., 2014). Our results showed that the intake of metabolizable energy increased while feeding in the terrestrial stratum increased, whereas PF was not significantly predictive. Furthermore, the positive relationship between ME and terrestrial feeding became stronger when the proportional difference between terrestrial and arboreal feeding became larger. This is the opposite for PF ratio, in that despite the general mean difference of food item values between these strata, its relationship with terrestrial feeding actually becomes weaker as the proportional difference between daily feeding in the terrestrial and arboreal strata becomes larger. It is possible that PF ratio was not as important given the bamboo lemurs' seasonally large proportion of fruits in their diet, for which PF is not an accurate measure of dietary quality (Wallis et al., 2012).
The challenge of meeting energy requirements is faced by many primates (for examples and reviews see, Irwin, Raharison, Raubenheimer, Chapman, & Rothman, 2014; Vogel et al., 2012), and perhaps is even more difficult in the tropics as plants in warmer climates generally have lower nutrient values compared to temperate plants (Chiy & Phillips, 1995). For example, higher mineral concentrations (e.g., sodium) in terrestrial foods are often associated with primate terrestrial feeding bouts (Campbell et al., 2005; Izawa, 1993; Link et al., 2011), thus primates likely only consume the minimum amount necessary to meet their needs (Rode, Chapman, Chapman, & McDowell, 2003). The large proportion of time spent feeding on the ground by Hapalemur in our study seems unlikely to be in response to reduced mineral concentrations, however, especially since the daily PF ratio and ME from terrestrial food items were of greater value compared to arboreal items. The location of our study, however, is an intricate matrix of upland littoral forest and swamps (Eppley et al., 2015a), and as swamp plants are often sodium-rich (Belovsky, 1981; Oates, 1978), it is possible that terrestrial grazing in the swamp may satisfy these needs.
4.3 Daily path length
Considering other folivorous primates, black and white colobus monkeys (Colobus guereza) from Kibale National Park in Uganda were shown to increase their DPL as foods became less abundant (Harris, Chapman, & Monfort, 2010). This is similar to the frugivorous white-bellied spider monkeys (Ateles belzebuth belzebuth), a species observed to travel greater distances in the wet season (Nunes, 1995), displaying longer daily paths when feeding trees are further apart from one another (Suarez, 2006). While our only seasonal and/or climatic factor shown to influence terrestrial feeding was colder temperatures, DPL similarly decreased as terrestrial feeding increased. This is likely due to the clumped, yet ubiquitous, distribution of graminoids throughout the Mandena littoral forest and swamp, allowing the lemurs to graze for longer periods of time in one area rather than spend time traveling between scattered food resources.
4.4 Predation risk
Predation pressure by arboreal and terrestrial species likely occurs at a similar rate (Shattuck & Williams, 2010), and may play a significant selective role—both proximately and ultimately—in the habitat use and positional behavior of arboreal primates (Gebo, Chapman, Chapman, & Lambert, 1994; McGraw & Bshary, 2002). Given that H. meridionalis display a cathemeral activity pattern (Eppley et al., 2015b), proximate fluctuations in predation risk may cause temporal niche shifts, changes in home range use, and/or the vertical strata (Gautier-Hion, Quiris, & Gautier, 1983; McGraw & Bshary, 2002). According to our model, canopy exposure did not influence increased terrestrial feeding. Furthermore, they largely grazed in swamp areas of densely distributed grasses and reeds, which would presumably result in closer neighbor proximities. Our results, however, do not support this.
The southern bamboo lemurs often traveled between the littoral forest and swamp habitats, which required terrestrial travel to cross the open gaps (Eppley et al., 2015a), potentially increasing their risk from both aerial and terrestrial predators. While neither anti-predator measure was supported in our model, H. meridionalis still maintained vigilance and alarm-called if they noticed a potential aerial or terrestrial threat (TME, personal observation). Examples include alarming and descending from the canopy when seeing an aerial raptor (e.g., Accipiter henstii, Buteo brachypterus, and Polyboroides radiatus), alarm-barking and ascending trees when encountering snakes (e.g., Acrantophis dumerili and Leioheterodon madagascariensis), and even alarm-barking and fleeing when encountering Eupleridae carnivores (e.g., Galidia elegans). Also during the study period, two feral dogs (Canis familiaris) were observed within Mandena and on three occasions we witnessed encounters whereby they chased grazing bamboo lemurs, forcing the group to ascend trees for protection (TME, personal observation). Similar to our observations, it has been reported that feral dogs have harassed northern muriquis (Brachyteles hypoxanthus) (Melo et al., 2005). Even more dire, both black-horned capuchins (Cebus nigritus) and brown howler monkeys (Alouatta guariba) have been reported being killed by feral dogs while traversing forest gaps terrestrially (Galetti & Sazima, 2006). While some arboreal species may experience increased predation pressure when shifting to a terrestrial niche (Newell, 1999; Xiang et al., 2009), terrestrial behavior by more ecologically flexible species may better facilitate movement and potential dispersal throughout a landscape, lessening the impacts of genetic erosion and habitat fragmentation (Ancrenaz et al., 2014; Laurance, 1990; Pahl, Winter, & Heinsohn, 1998). One confirmed successful act of predation was recorded among our groups of bamboo lemurs; using radio telemetry, we discovered a male Dumeril's boa (Acrantophis dumerili) had preyed on an adult female H. meridionalis from group 1. The large terrestrial boa was located in a vast swamp area where the bamboo lemurs often feed terrestrially, thus it is likely that she was captured while on the ground (Eppley & Ravelomanantsoa, 2015). Despite this, bamboo lemurs were observed to occasionally sleep on the ground for long periods of time (Eppley, Donati, & Ganzhorn, 2016a), potentially lending support for an overall reduced risk of predation within Mandena.
Bamboo lemurs maintain morphological adaptations, that is, short arms and proportionally long legs (Jungers, 1979), for vertical clinging and leaping, their primary mode of locomotion; however, Hapalemur often move quadrupedally along branches while foraging (Fleagle, 2013), allowing them to extend their niche to the terrestrial stratum. Similarly, indriids are also known for their vertical clinging and leaping (Jungers, 1979), and occasionally descend to the ground to travel and forage (Irwin et al., 2007; Pollock, 1975; Richard, 1974). In the dry, spiny forest of southwest Madagascar, Verreaux's sifaka (Propithecus verreauxi) are highly vigilant when foraging terrestrially and/or consuming soil, and respond to terrestrial predators by quickly ascending trees (Brockman et al., 2008), an anti-predator behavior also observed in Milne-Edward's sifaka (P. edwardsi) and red-fronted lemurs (E. rufifrons) within the eastern humid forest (Overdorff et al., 2002). These sympatric lemurs often fed in riskier locations compared to red-bellied lemurs (E. rubriventer), an observation attributed to their comparatively larger group sizes (Overdorff et al., 2002). Correspondingly, small groups of ring-tailed lemurs (L. catta) have been shown to avoid terrestrial feeding, unlike larger groups, when the perceived risk of predation was high (Gould & Sauther, 2007). Similar to Malagasy strepsirrhines, Neotropical primates are well-known for their arboreality, yet many spend at least some time on the ground, for example, Alouatta spp. (Bicca-Marques & Calegaro-Marques, 1995; Pozo-Montuy & Serio-Silva, 2007), Ateles spp. (Campbell et al., 2005), Brachyteles spp. (Dib, Oliva, & Strier, 1997; Mourthé et al., 2007; Tabacow, Mendes, & Strier, 2009), Cebus capucinus (Gilbert & Stouffer, 1995), and some pitheciin monkey genera (Barnett et al., 2012). These observations of terrestrial behavior are often associated with disturbed habitats whereby animals traverse open areas between forest fragments, potentially increasing their exposure to predators (Campbell et al., 2005; Takemoto, 2004). Unlike southern bamboo lemurs, which readily descended to the ground without hesitation or prolonged vigilance (TME, personal observation), spider monkeys appear very nervous when terrestrial, continually scanning the area and taking long periods of time before fully descending (Campbell et al., 2005), in addition to maintaining closer nearest neighbor proximities when exposed (Di Fiore, 2002). In fact, a multi-site analysis found that in sites with more intact predator communities (i.e., greater perceived risk of predation), spider monkeys only occasionally (≤ 5% of sampling) fed on the ground when nutritional returns were high (Campbell et al., 2005).
4.5 Additional costs
The utilization of a terrestrial dietary niche likely imposes additional costs on bamboo lemurs. The gastrointestinal tract of Hapalemur spp. certainly assists in their elevated ability to digest fiber, allowing for leafy material to be fermented by symbiotic gut microbes (Campbell, Eisemann, Williams, & Glenn, 2000; Perrin, 2013). While this likely allows for digesting the large quantities of graminoids in their diet, feeding on grasses is often associated with the evolution of several dental modifications (Yamashita, Vinyard, & Tan, 2009), mostly due to the abrasive silicates, that is, phytoliths, that are embedded in the epidermal layer of grass leaves (Judziewicz, Clark, Londoño, & Stern, 1999). These have the potential to increase the rate of wear on teeth through the mastication of this abrasive vegetation (Cuozzo & Yamashita, 2007; Jablonski, 1994; Kaiser et al., 2013; Yamashita et al., 2009), which may lead to a more rapid dental senescence. It is also possible that increased terrestriality may increase exposure to unfamiliar pathogens (Anderson, 2000; Zommers, Macdonald, Johnson, & Gillespie, 2013), thus increasing parasite loads compared to sympatric arboreal species (Loudon & Sauther, 2013). In collared brown lemurs, endo-parasite prevalence has been shown to be higher in the degraded area of Mandena compared to more intact fragments of littoral forest (Lazdane et al., 2014). While we have hypothesized that this species' use of visually conspicuous latrines may act to limit the spread of feces throughout their territory, we have no precise way of testing this (Eppley et al., 2016b).
5 CONCLUSION
Considered in whole, our results suggest that the initial expansion to a terrestrial dietary niche may have occurred when the nutritional pay-off was greater in the new stratum. This observed flexibility and use of a terrestrial dietary niche is likely to be an adaptation to a habitat devoid of their primary food resource, that is, bamboo, which southern bamboo lemurs are often found near and feeding on at other sites, for example, Andohahela NP (Feistner & Schmid, 1999; Fausser et al., 2002). In the absence of these foods, H. meridionalis in Mandena appear to have greatly expanded their dietary diversity while utilizing a terrestrial feeding niche. Terrestrial feeding was also influenced by the distribution of resources, and appeared to provide a thermoregulatory benefit. Interestingly, the lemurs' perceived predation risk (as shown through canopy exposure and nearest neighbor proximity) had no influence. Contrary to the idea that only large primates evolved terrestriality, the bamboo lemurs' small body mass and morphological adaptations suited for arboreality (Fleagle, 2013; Jungers, 1979) provides us a window into the ultimate determinants of niche expansion: ancestral primates, in the absence of their primary resources, may have initially descended in peripheral population range areas where the benefits out-weighed the costs. Furthermore, our data present strong evidence for the ability of this species to subsist in anthropogenically-disturbed environments, demonstrating that they may be more flexible than some of their congeners (e.g., H. alaotrensis, H. aureus). In general, these lemurs are highly adaptable and do not have rigid dietary restrictions, rather they appear to cope well within a seasonal and ever-changing landscape.
ACKNOWLEDGMENTS
This work was carried out under the collaboration agreement between the University of Antananarivo and the University of Hamburg, as well as a collaboration agreement between TME and QIT Madagascar Minerals (QMM). We thank the Direction du Système des Aires Protégées, and the Ministère de l'Environnement et Forêts of Madagascar for permission to conduct research. Special thanks to Jacques Rakotondranary and Tolona Andrianasolo for research permit acquisition and logistical assistance, to Katie Hall and Natalie Breden for their assistance in the field, and to Julia Watzek for assistance with the statistical analyses. We also thank the Environmental Team at QMM Rio Tinto for their assistance and provision of on-site logistical support and acknowledge their helpful staff, especially Jean-Baptiste Ramanamanjato, Johny Rabenantoandro, Faly Randriatafika, Laza Andriamandimbiarisoa, David Rabehevitra, and Robertin Ravelomanantsoa. We are grateful to Irene Tomaschewski for plant biochemical analyses. We thank associate editor and reviewers for their constructive comments that improved the quality of this manuscript.




