The taphonomy of blood components in decomposing bone and its relevance to physical anthropology
ABSTRACT
Objectives
The variation and persistence of blood components, in particular red blood cells (RBCs), within bone tissue during the decomposition process, especially at the early stages and in different taphonomic conditions, has never been thoroughly investigated, regardless of the fact that knowing how blood survives or degrades within bone could be of help in solving many anthropological issues, such as trauma analysis and interpretation.
Materials and Methods
This research investigated the influence of time and taphonomy on the persistence and detectability of blood components in parietal bone fragments (of different post mortem periods and taphonomic conditions) through histological (Hematoxilin and Eosin, HE) and immunohistochemical (Glycophorin A, GYPA) analyses.
Results
The immunohistochemical investigation for GYPA showed the presence of RBCs under the form of erythrocyte debris or residues otherwise morphologically unidentifiable using only HE staining. Hence, while well-defined RBCs can be observed only in the first week of decomposition, afterward these structures can be detectable with certainty only by immunohistochemical analysis, which reveals discrete quantities of RBC residues also in dry bone (post mortem interval, or PMI, of 15 years), but not in archaeological samples, in which the greater PMI and the different taphonomic conditions together could be the answer behind such difference.
Discussion
This study highlights the usefulness and potential of immunohistochemical detection of GYPA in RBC investigation and gives a realistic idea of the persistence and detectability of erythrocytes in different osteological taphonomic conditions, in contrast to results reported by some authors in literature. Another important result concerns the detection of RBC residues in dry bone, which opens the way to the possible use of RBCs in trauma interpretation. Am J Phys Anthropol 158:636–645, 2015. © 2015 Wiley Periodicals, Inc.
After death, the body is exposed to different endogenous and exogenous variables that affect the progression of postmortem modifications, but also the appearance and structure of body, tissues, cells, and biomolecules. In literature many articles show how the body changes and appears at different decomposition stages (fresh, bloated, decay, post-decay, and skeletal) and in different environments (Kovarik et al., 2005; O'Brien and Kuehner, 2007; Voss et al., 2008; Lee Goff, 2009), whereas a study already dealt with how bone varies in various taphonomic conditions: e.g. bleaching is associated with sun exposure, whereas rarefaction with mechanical erosion (Quatrehomme and Işcan, 1997).
Always referring to the microscopic and biomolecular level, another important factor is how the organic and inorganic components of bone tissue react and change over time after death; Dent et al. (2004) showed how protein degradation and loss of mineral hydroxyapatite take place and emphasized the link between bone preservation and burial environment.
Unlike the above mentioned macroscopic and microscopic changes on bone tissue caused by environmental factors, scavengers and microorganisms, which have already been explored by the anthropological literature (Marchiafava et al., 1974; Hackett, 1981; Jans et al., 2004), little is known about bone soft tissue degradation and its components, particularly the cellular ones. This is of crucial importance since specific cellular populations surviving in bone, on fractures, or within a particular lesion, may be diagnostic of the timing of a fracture, or may be markers of a specific disease. Archaeological and anthropological literature have not yet approached this issue in a critical fashion, meaning that sometimes gross misinterpretation of the cellular structure on archaeological or forensic bone can be seen or read.
Bone tissue is formed by a protein structure (mainly collagen), strengthened by a mineral constituent (hydroxyapatite), and other organic components (e.g. mucopolysaccharides and glycoproteins) (Goffer, 1980; Janaway, 1997; Hare, 1988). Like every other tissue in the body, the bone tissue is characterized by vascularization and therefore it houses in its structures all the cells and typical components of the blood: erythrocytes, leukocytes, platelets, albumin, fibrinogen and other plasma proteins. These components are ubiquitous in the bone tissue: specifically, they can be observed at the periosteum (within the vessels located here), inside Haversian and Volkmann canals (transverse or oblique canals that connect the meaning functional bone units, the so-called osteons), located in both lamellar and trabecular bone, in vessels placed into bone marrow spaces where hematopoiesis takes place, and therefore where blood cells can be found at various stages of maturation.
All of these components may be useful for understanding microscopic changes occurring within bone tissue during the decomposition process and in relation to different environmental conditions in which a body decomposes (water, high or low temperature, humidity, air). In this regard it would be interesting to know which variations take place (e.g. in the persistence and morphological changes of blood components) and in which specific structures.
In literature histological knowledge on bone tissue blood and marrow components is limited to diagnostic histopathology or paleopathology (van den Berg et al., 1990; Thiele et al., 2000; Schultz, 2001; Thiele et al., 2001; Wain et al., 2001; von Hunnius et al., 2006; Wilkins et al., 2008; van der Merwe et al., 2010; Schultz, 2012). In the forensic field, Penttilä and Laiho (1981) investigated the morphological changes of blood components (such as erythrocytes, white blood cells, and platelets) in cadaver blood at different post-mortal intervals; their results highlighted modifications in shape and size over time. Differently, other studies focused on morphological changes of blood cells over time with the intent of aging them and supplying possible information useful in the estimation of time since death (Dokgöz et al., 2001; Chen and Cai, 2006; Strasser et al., 2007; Wu et al., 2009). Finally, attention was also given to possible mistakes in differential diagnosis between erythrocytes and spores and pollens (Cappella et al., 2015). Nevertheless, the observation of changes of blood components within bone tissue with regard to decomposition processes has not yet been fully explored: for how long are well-preserved blood components observable in bone tissue of cadavers and still detectable over time, in which area of bone tissue are they mostly protected or unaltered, and again what information can be gained from their persistence in relation to different environmental conditions, all questions that still remain unanswered. In other words, when we look at something that looks like a cell in old or ancient bones, is it really what we think we are looking at? Regardless of the caution which should be adopted in answering this question, in literature authors have report the presence of erythrocytes or structures like-erythrocytes in ancient skeletal remains (Stout and Teitelbaum, 1976; Penttilä and Laiho, 1981; Maat and Baig, 1990; Maat, 1991; Schweitzer et al., 2007; Cappella et al., 2015), but in most of these cases such findings have not yet been demonstrated by the specific techniques that science owns.
In this perspective, the current study aims to examine the changes and persistence of blood components (with special attention to erythrocytes) in bone tissue in relation to different decomposition and taphonomic conditions over time, by using histology (Hematoxylin-Eosin staining, HE) and immunohistochemistry (Glycophorin A, GYPA). The observation concerns histological assessments conducted on bone samples from both well and badly preserved cadavers (early decomposition), as well as from modern and ancient skeletonized remains, in order to observe how blood components appear, if they are noticeable, and for how long their persistence can be detected in such conditions. The present study may contribute to clarify the use of some valid techniques as an aid to the understanding of taphonomy of microscopic bone structures in skeletal remains and acquiring new important information useful in the field of paleopathology and trauma analysis in human remains, possibly demonstrating also the presence of bleeding in trauma, an aspect that has never been investigated in this perspective.
MATERIALS AND METHODS
Bone samples
The study was conducted on a total of 32 samples of parietal bone fragments, which had been previously sampled from forensic cases from eight different cadavers (with no blood diseases or bone pathological conditions reported) at the Institute of Legal Medicine of Milan, according to Police Mortuary Regulations. The cadavers were characterized by different states of decomposition (early and advanced): the diverse post mortem intervals (PMI) as summarized in Table 1.
| Sample n° | Decomposition degree | PMI |
|---|---|---|
| 1 | Fresh cadaver | <24 hrs |
| 2 | Fresh cadaver | <24 hrs |
| 3 | Fresh cadaver | <24 hrs |
| 4 | Fresh cadaver | <24 hrs |
| 5 | Putrefied cadaver | 48< hrs < 72 |
| 6 | Skeleton | 20 yrs |
| 7 | Skeleton | 20 yrs |
| 8 | Skeleton | 400 yrs |
The parietal bone fragments from the three well-preserved cadavers (samples 1, 2 and 3, all with a PMI <24 hrs) were exposed to decomposition in air at standard environmental conditions (constant humidity and temperature), and then monitored weekly for the two following months (for a total of eight fragments per sample). Unlike the previous, sample 4 (derived from a well preserved cadaver with a PMI <24 hrs) was divided in fragments subjected to diverse laboratory procedures in order to simulate various taphonomic contexts, consisting in boiling (for 2 hrs), maceration (immersion in cold water for 1 month) and freezing (−4°C for a month). From every sample mentioned above, a small portion was used as a control for the evaluation of all the experiments, and therefore was not subjected to any experimental conditions. All these information are summarized in Table 2.
| Time of air decomposition (wks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample n° | T0 (ctrl) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
| 1 | <24 hrs | 1 wk | 2 wks | 3 wks | 4 wks | 5 wks | 6 wks | 7 wks | |
| 2 | <24 hrs | 1 wk | 2 wks | 3 wks | 4 wks | 5 wks | 6 wks | 7 wks | |
| 3 | <24 hrs | 1 wk | 2 wks | 3 wks | 4 wks | 5 wks | 6 wks | 7 wks | |
| Laboratory treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample n° | 1 | 2 | 3 | 4 | |||||
| 4 | Ctrl | Freezing | Boiling | Maceration | |||||
- While sample 1, 2, and 3 were subjected to decomposition in air, sample 4 was subjected to laboratory treatment for simulating different taphonomic conditions.
In addition, parietal fragments were taken also from human remains in which the decomposition process of skeletonization occurred in real conditions, in order to compare results obtained from actual circumstances and the experimental setting. The real conditions are represented by a cadaver at the early stages of putrefaction (sample 5, with a PMI ranging between 2 and 4 days) and in skeletonized remains of different PMI (sample 6, 7, and 8). In particular, samples 6 and 7 were obtained from two well-preserved modern skeletons (selected from the “Milano Skeletal Collection,” a modern referenced skeletal population) (Cappella et al., 2014), while sample 8 was taken from a badly preserved ancient skeleton (1600–1630 AD).
Histological and immunohistochemical analysis
The same protocol was used for preparing all bone fragments for both histological and immunohistochemical analysis, consisting in fixation (in 10% Formalin for 24 hrs) and decalcification (in a decalcifying solution containing 14% hydrochloric acid, DECALC, Histo-Line Laboratories, Milan). The decalcification was performed at room temperature for a time dependent on the size and thickness of the fragment (ranging from 4 hrs for ancient bone fragments to 2 days for fresh bone samples). Once the optimal flexibility was achieved, the samples were washed up under running tap water for 24 hrs before proceeding with dehydration and embedding into paraffin. From each sample treated as described, 5 micron sections were obtained and subsequently stained with Hematoxylin and Eosin (HE) for standard histological analysis, as well as immunohistochemically stained for the identification of Glycophorin A (GYPA), a specific membrane antigen for erythrocytes. The latter was carried out using a monoclonal antibody (antihuman Glycophorin A, clone JC159, Dako-Dakopatts, Denmark) diluted 1:400 by a Dako Autostainers, as according to the literature (Luna, 1968; Cattaneo et al., 2010).
Microscopic evaluation
Histological analysis and immunohistochemistry allowed us to observe in detail the blood components of every single bone tissue structure localized in the different layers: periosteum, outer compact bone, diploe (spongy bone), and inner compact bone. Specifically, the analysis focused on the research of blood components within all structures such as Haversian and Volkmann canals, canals and vessels localized in bone trabeculae, and in bone marrow spaces (Fig. 2).
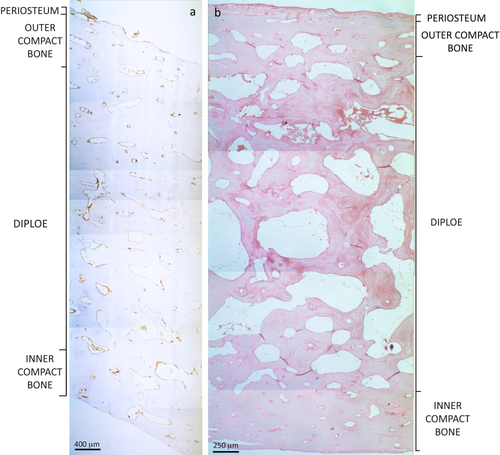
Examples of thin parietal sections stained by anti-Glycophorin A (a) and Hematoxilin-Eosin (b) in which it is possible to observe the division of the different layers (several merged photomicrographs at 40× magnification). The score of RBC-related structures (such as isolated, agglomerated, organized in rouleaux, and/or eosinophilic accumulation) was performed in each vessel, canal, and marrow space of each layer within the entire section and then calculated as the amount of structures in which RBCs are localized. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
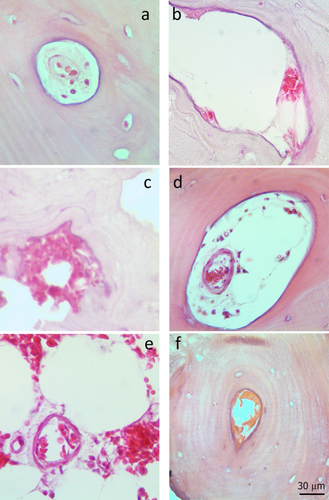
Example of some parameters used as a score in the microscopic evaluation: a) Intracanal dispersed erythrocytes in a Haversian canal; b) Intracanal accumulated erythrocytes; c) Extracanal accumulated erythrocytes; d) Intravasal agglomerated erythrocytes into a Haversian canal; e) Intravasal erythrocytes in rouleaux into a marrow space; f) Intracanal aspecific accumulations. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The score used for the evaluation (in structures of every layer) included different parameters concerning red blood cells and not merely their presence/absence: the erythrocytes, if present, were considered as dispersed/isolated, organized in rouleaux, agglomerated, and as generic eosinophilic accumulation which could be (but not necessarily) related to decomposing RBCs; the presence of nucleated cells was also indicated, when recognizable. Moreover, as additional information, the position of cells with respect to the vessels and canals were inspected: RBCs were described as intra- or extra-vasal when concerning vessels, intra- or extra-canal if regarding Volkmann and Haversian canals, or indeterminate in case of impossibility in identifying their origin (Fig. 3).

a) Sample 2 at T2, showing numerous Haversian canals full of blood components (100× magnification); in this case the histological analysis permits to highlight only a nonspecific accumulation, as that shown in b) at a greater magnification (200×), in contrast with the well-defined erythrocytes visible at T0, as shown in c) (at 400× magnification). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Afterwards, a semi-quantitative assessment was possible by calculating the percentage of canals and bone marrow spaces containing red blood cells. In particular, a ratio between the total amount of structures in which blood elements in theory should have been present (e.g. vessels, marrow spaces, etc.) and the total number of structures where blood elements actually were present was calculated, in order to obtain percentages representing those structures in which RBCs or cellular hematic debris could still be detected. For all samples both histological and immunohistochemical evaluations were performed in order to confirm the results. When the histological observation did not allow for the certain identification of well-defined cells (scored as described below), but only a strong eosinophil accumulation was detectable (referred to as hematic components by the authors, as confirmed by immunohistochemical analysis), the term “nonspecific accumulation” was used.
Finally, our first objective was to analyze how the features of red blood cells in bone tissue change in the monitored samples at the early stages of decomposition in air (for a maximum of 2 months). In this case the evaluation concerned the quantity of structures containing blood with respect to the total canals, vessels, and marrow spaces, but also the recognition of single/groups of defined red blood cells before their decomposition and disappearance. The same assessment was conducted also on all those samples in which the real decomposition process had already started a few days before (sample 5) and on those for which the natural skeletonization process had occurred over a period of years or centuries (sample 6, 7, 8).
The subsequent examination of all samples by immunohistochemistry had the specific purposes of identifying red blood cell residues as fragments of RBCs when they are no longer morphologically recognizable, or, on the other hand, of dismissing other debris as non-RBC.
Microscopic observation was performed by an optical microscope (LEICA DMLB) at several magnifications (4×, 10×, 20×, 40×, 63×) with 10× eyepieces. By means of a photographic system (Eurekam 3.0, DV-3000), photographic surveys related to the most significant areas were captured.
RESULTS
Results from HE staining revealed well defined red blood cells only in control samples (1, 2, 3 at T0 and 4, not treated) as well as in sample 5 (fragment taken from putrefied cadaver with PMI between 48 and 72 hrs). In other words, red blood cells were no longer observable as cells after a week of decomposition in air: from this time onward solely nonspecific accumulations were noticeable by using HE staining (Fig. 4).
a) Sample 2 at T3, displaying numerous Haversian canals still full of hematic components, indicated by black arrows (40× magnification). In this case the immunohistochemical analysis allows us to prove the presence of the erythrocytes in the nonspecific accumulation or in those cases where the RBC morphology is not clearly identifiable as such: in fact, the dark brown coloration means that the antigen GYPA is located into the canals and vessels, therefore proving the hematic origin also for those nonspecific accumulations highlighted by the histological analysis. In b), a Haversian canal of sample 3 at T4 at greater magnification, in which some preserved erythrocytes are still recognizable, as those indicated by black arrows (400×). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
On the contrary, the immunohistochemical analysis adopted for the search of GYPA allowed for the detection of the presence of red blood cells even when degraded (no defined intact cells but only hematic debris), otherwise not detectable by the histological examination; thus, this analysis allowed us to confirm the hematic nature of most nonspecific accumulations located into canals and vessels stained by HE (Fig. 5).
a) Sample 1 at T1 shows numerous Haversian canals completely full of blood components, stained by HE (general view at 40× magnification). In this case, the canals do not need to be indicated by arrows as they are very well observable even without any help (an enlargement is visible on the right, at 630× of magnification). In b) a total view (40× magnification) of sample 1 at T6: despite the bone fragment has decomposed for about 1 month and a half, Haversian canals still have blood components inside in a high percentage out of the total of these structures, as for sample 1 at T7 (1 week later), shown at 40× of magnification in c). In both, b) and c) the nonspecific accumulations located in the canals are indicated by black arrows in order to help the observer in their identification, and an enlargement (630×) is proposed on the right, for a better visualization of the characteristics described. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Regarding the persistence of blood components (assessed as the percentage of bone tissue structures containing RBC), no great changes in terms of percentage were observed concerning the passing of time in the first 2 months of decomposition in air; the percentages obtained by the evaluation in control samples are similar to those found in samples at all experimental times and also after 2 months circa of decomposition in air (Fig. 6). The differences found concern mostly the abundance of coloration: if at very early times, like T0, T1, and T2, the canals are completely full of components, at a more advanced time the same structures show a lighter staining and little space is occupied by the components (Fig. 6).
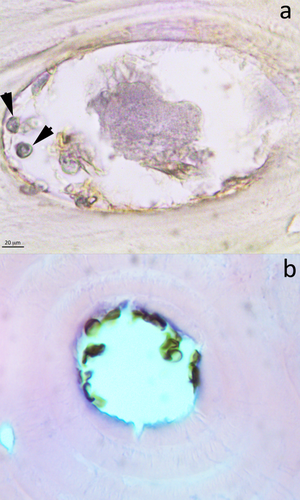
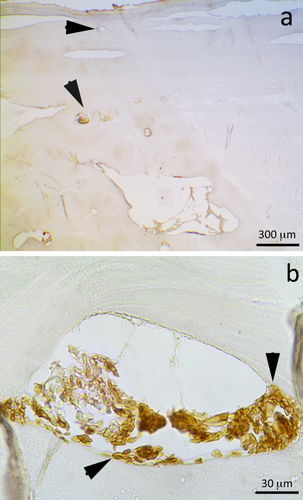
Sample 6, derived from cemetery skeletal remains (PMI of 15 years) stained by HE (left image at 40× magnification) and by GYPA staining (right image at 100× magnification). Only the immunohistochemical analysis permits to observe the presence of blood component residues (indicated by black arrows in the right image). The histological analysis, in fact, shows only the presence of some brown structures, which can resemble erythrocytes in terms of morphology and size, but, since they are not stained by Eosin as the components in all the other samples (staining includes nonspecific accumulation and defined blood components), their origin is supposed to be botanical or from some other contamination. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Hematoxilin and Eosin revealed percentages between 60% and 80%, also confirmed by GYPA, which demonstrated percentages slightly higher, but very similar. As a matter of fact, the immunohistochemistry in general showed higher values compared to the common histological analysis, revealing in this sense not only the presence of entire cells, but also RBC fragments in samples at more advanced stages, not detectable by HE (percentages around 80% in cases where HE revealed just 60%) (Graph 1).
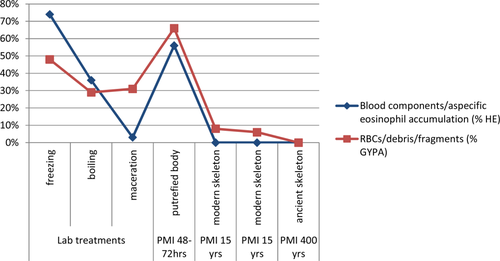
Percentages relative to the number of tissue structures (e.g. vessels, marrow spaces, etc.), among the total amount present, in which blood components and their debris could actually be detected. The slides of the cases subjected to laboratory treatment (Sample 4), the real putrefaction case (Sample 5), and the skeletonized ones (Samples 6–8) are considered: each sample has been analyzed in toto, relating the number of those structures in which blood components or RBC debris were present to the total number of bone and vascular structures observable (e.g. Haversian and Volkmann canals, vessels, and marrow spaces). The blue line refers to the histological analysis (HE), whereas the red one refers to the immunohistochemical staining (GYPA). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Similar results were found also in real putrefaction case (sample 5, PMI 48–72 hrs), where well-defined cellular elements as well as abundant nonspecific accumulations are represented by percentages greater than 60% in both histological and immunohistochemical analysis. Unlike the previous case, the detection of RBC in skeletonized human remains samples characterized by a higher postmortem interval (20 years, 6 and 7) was possible only by using the monoclonal antibody GYPA. Hematoxilin and Eosin did not allow to observe either well defined blood components nor nonspecific accumulation, but only generic debris (which could be hypothetically the result of putrefied products as well as both botanical and microorganism contamination, as displayed in Fig. 8 and 9), while the modern skeletal remains showed RBC residues in more than 10% of canals and marrow spaces (Fig. 6), the ancient skeleton (sample 8) shows a complete lack of any cellular debris, as confirmed by GYPA stained sections (Graph 1).
Concerning the results from the evaluation of samples subjected to lab treatments, both the analyses revealed a great similarity between the control and the frozen sample, which displayed both erythrocytes and nonspecific accumulations in consistent percentages (>50%), similar to what is seen in the control (60%). If on one hand the presence of blood components is maintained quite constant in the frozen sample (Fig. 7), on the other hand the reduction of blood components (values close and under 30%) has been emphasized in the boiled and macerated sample. In this last case solely the immunohistochemical investigation stressed the RBC presence in canals and marrow spaces (Graph 1).
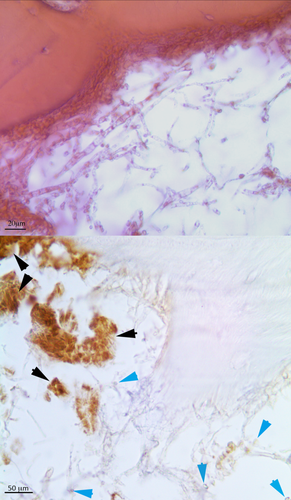
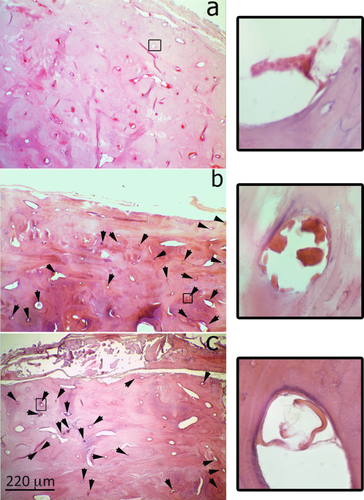
a) Sample 4–2 (freezing treatment) stained by HE (400× original magnification). The erythrocytes are well localized everywhere in cortical bone in the Haversian Canals and into marrow spaces. The treatment seems to maintain constant the level of the components. b) and c): Images depicting the same structure (630× original magnification) stained by HE (b) and GYPA (c): despite the different staining technique, the same hematic elements are well-identifiable, therefore proving, thanks to GYPA, that the elements highlighted by HE are actually RBCs. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
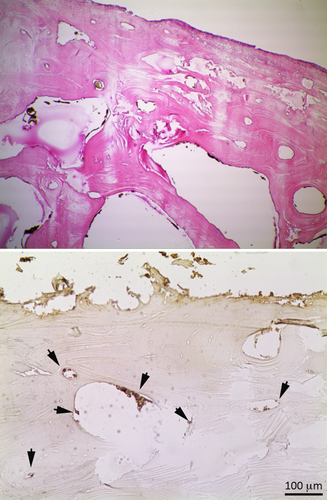
a) Sample 2 (T5) stained by GYPA (630× original magnification). The black arrows in a Haversian canal indicate some erythrocyte like-structures; the dimension together with the absence of any immunostaining reaction indicate that a hematic origin is very unlikely and rather suggest microorganism contamination. b) Similar structures stained by HE (630× magnification) resembling erythrocytes whose antigenic nature was then not confirmed by GYPA staining (a). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
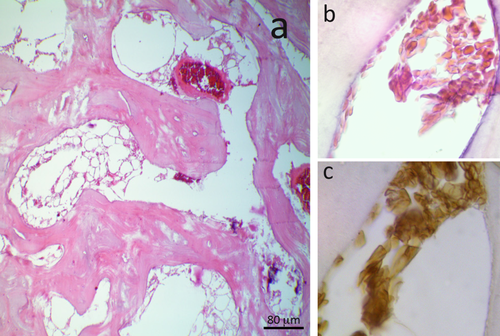
The images show an example of microorganism contamination in a marrow space: the HE staining makes it difficult to interpret the section, whereas with the immunohistochemical technique it is possible to differentiate the hematic debris (stained in marrow and indicated by black arrows) from other non hematic origin materials/structures (light blue arrows). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Given the poor knowledge concerning decomposition and taphonomic effects on blood components in the bone tissue, the present study focused on the acquisition of such information through histology and immunohistochemistry. Decalcified sections of parietal bone fragments, characterized by different states of decomposition and various postmortem intervals, were analyzed with the intent to observe differences due to various PMI and taphonomic variables. An evaluation of the presence/absence of erythrocytes or of their residues was considered in all canals and marrow spaces of the sections, and so their respective percentages. The immunohistochemical technique, by using antibodies for GYPA, made it possible to highlight data otherwise not noticeable in the sections stained by HE: in fact, it allowed us to observe whether the so-called nonspecific accumulations were of RBC origin or not. Obviously, they may contain also other cellular and hematic debris, which still have to be ascertained with further specific immunohistochemical investigations.
Results from both analyses demonstrated that cells appear well defined only if the period of time is shorter than 1 week (in control sample and in sample 5), a fact that suggests a certain influence of time on the preservation of morphology, as reported in a recent publication (Cappella et al., 2015). If the morphological preservation of RBCs is quite dependent on time (since the very early stages of decomposition), on the contrary the debris were seen to survive longer than expected: the percentage of bone tissue structures in which such cell remains are still detectable, especially by immunohistochemistry, is constant in the first 2 months of decomposition in air. The confirmation of these results comes also from the assessments conducted on samples taken from a cadaver in putrefaction (PMI less than a week), representative of a real decomposition circumstance, in which biconcave red blood cells are still well identifiable and observed in considerable percentages of vessels and canals.
Also in modern skeletal remains with a PMI of 20 years RBCs are traceable (even if in very low quantities). In these last samples, in fact, despite the long time elapsed since death, a small percentage of canals and marrow spaces (10%) still contains RBCs, which were only discernable by immunohistochemistry; on the contrary, a total loss of these cells was observed in samples with PMI in the order of centuries (sample 8). This last account revealed divergent results from findings of some recent publications (Maat and Baig, 1990; Maat, 1991; Schultz, 2006; Schweitzer et al., 2007; Charlier et al., 2008; Setzer et al., 2013), where the identification of red blood cells even in ancient remains and through different techniques is frequently reported. Despite the present study did not investigate conditions equal to those reported in some publications, such as fossilization or mummification (conditions in which the bodies may be exceptionally well preserved), the authors observed great difficulties in recognizing well defined cells after a week of decomposition, and in most samples this was provided just because of the implementation of immunohistochemistry. The crucial point in this sense is obviously the technique used to demonstrate the presence of red blood cells, which has to be based not only on morphology but also on more satisfactory requirements at the biomolecular level. In this sense the suggestion is towards caution. In fact, the structures of botanical nature sometimes can be very tricky and may mimic the presence of erythrocytes, as reported by a recent work (Cappella et al., 2015), which shows the similarity between red blood cells and spores/pollen as well as providing important information about the survival and morphological modification of erythrocytes in vitro.
The results obtained from samples subjected to maceration, boiling and freezing showed that the normal laboratory practices, commonly required for cleaning up bones (maceration and boiling), remove most of the cell components of the tissue, even though some minimal quantity of debris can survive in it, therefore being detectable only by immunohistochemistry. On the contrary, the conservative procedures, such as freezing, revealed good conservation of cellular components, not only in terms of presence and quantity, but even in terms of quality (by preserving the morphology). Moreover, in the frozen sample the presence of some cracks caused by the thermal shock, which can cause migration of nonspecific accumulations or debris, was also detectable. The evaluation of the percentages of bone structures containing blood elements allowed, not only to verify the persistence of blood components, but also to highlight the sensitivity of the techniques exploited. In fact, in those cases in which the HE staining did not provide clear results, the immunohistochemical analysis showed the presence even of the smallest blood group elements and hematic debris thus proving to be a reliable method for the study of hematic components also in decomposed material.
In general, the study was based on experimental conditions (control samples and samples from real conditions—putrefied cadaver and skeletal remains—apart) which simulate a process of decomposition, otherwise not easily comparable to what happens in the real decomposition of a body (as in a closed system like a cadaver): in the first case, it is the external lamina to be directly in contact with the surrounding environment; in the second, however, events concern the most superficial tissues that cover and protect the underlying bone tissue at the early stages of decomposition. This fact could give rise to some variations in the detection of blood components and in their survival in decomposed or decomposing bone tissue, although the potential of a technique such as immunohistochemistry in proving their presence and in being an optimal technique for the detection of blood components residues, even when they are just in traces, does not change. This study highlights the usefulness of immunohistochemical detection of GYPA in RBC investigation and gives an idea of the persistence of erythrocytes in different taphonomic and PMI conditions, often in contrast with results reported by some authors in literature, as mentioned above. The presence of RBC, as defined cells or as cellular residues, can be a possible marker not only in relation to PMI, taphonomic conditions, and decomposition, but also of bleeding or trauma in bone. This study is of course only a first approach to blood taphonomy in bone and it cannot be considered as a representative study for all the different conditions. Nevertheless it is a good starting point for future research, which needs to be conducted in order to acquire knowledge in this field. Nonetheless, the great capacity of the immunohistochemistry in searching for specific biomarkers in both fresh and dry bone tissue has emerged; the use of biomarkers such as RBC through immunohistochemistry could be crucial for forensic purposes such as the interpretation of PMI (analogous use of Luminol) or of trauma (for the first stages of healing or for the interpretation of vital lesions), all topics that still need to be deeply investigated and clarified in the anthropological field.




