Talar morphology, phylogenetic affinities, and locomotor adaptation of a large-bodied amphipithecid primate from the late middle eocene of Myanmar
Abstract
A well-preserved fossil talus [National Museum of Myanmar Primates (NMMP) 82] of a large-bodied primate is described from the late middle Eocene Pondaung Formation of central Myanmar. The specimen was collected at Thandaung Kyitchaung, a well-known amphipithecid primate–bearing locality near the village of Mogaung. NMMP 82 adds to a meager but growing sample of postcranial remains documenting the large-bodied primates of the Pondaung Formation. This new talus exhibits a suite of features that resemble conditions found in living and fossil haplorhine primates, notably anthropoids. As such, the phylogenetic signal deriving from the morphology of NMMP 82 conflicts with that provided by NMMP 20, a partial skeleton (including a fragmentary calcaneus) of a second large-bodied Pondaung primate showing undoubted adapiform affinities. Analysis subtalar joint compatibility in a hypothetical NMMP 82/NMMP 20 combination (talus/calcaneus) reveals a substantial degree of functional mismatch between these two tarsal bones. The functional incongruence in subtalar joint morphology between NMMP 20 and NMMP 82 is consistent with the seemingly divergent phylogenetic affinities of these specimens, indicating that two higher level taxa of relatively large-bodied primates are documented in the Pondaung Formation. On the basis of its size and morphology, we refer the NMMP 82 talus to the large-bodied amphipithecid Pondaungia. The occurrence of anthropoid-like tali in the Pondaung Formation obviates the need to invoke homoplasy to explain the shared, derived dental characters that are common to amphipithecids and undoubted anthropoids. Functionally, the NMMP 82 talus appears to have pertained to a primate that is engaged in active quadrupedalism in an arboreal environment along broad and subhorizontal branches. The primate taxon represented by NMMP 82 was capable of climbing and leaping, although it was not particularly specialized for either of these activities. Am J Phys Anthropol 143:208–222, 2010. © 2010 Wiley-Liss, Inc.
The Amphipithecidae is an extinct family of primates whose representatives evolved in South Asia during the early Cenozoic. They were first described in the early part of the 20th century from the late middle Eocene Pondaung Formation of Myanmar (Pilgrim, 1927; Colbert, 1937). Subsequent field research has documented a wider geographic and stratigraphic range for amphipithecids, including the late Eocene Krabi Formation of Thailand (e.g., Chaimanee et al., 1997; Ducrocq, 1999) and the early Oligocene Chitarwata Formation of Pakistan (Marivaux et al., 2005; Marivaux, 2006). The fragmentary nature of the amphipithecid fossil record, which consists of isolated teeth, jaws, and a few postcranial elements, has engendered considerable confusion and debate since their initial discovery. Indeed, the longstanding hypothesis that amphipithecids are an early radiation of Asian anthropoids remains one of the most contentious subjects in primate paleontology (e.g., Simons, 1971; Szalay, 1972; Ba Maw et al., 1979; Ciochon et al., 1985, 2001; Ciochon and Holroyd, 1994; Ducrocq, 1998, 1999, 2001; Godinot, 1998; Jaeger et al., 1998, 1999, 2004; Chaimanee et al., 2000; Takai et al., 2001; Beard, 2002, 2004; Ciochon and Gunnell, 2002, 2004; Gunnell et al., 2002; Shigehara et al., 2002; Marivaux et al., 2003, 2008; Takai et al., 2003; Chaimanee, 2004; Kay et al., 2004a,b; Shigehara and Takai, 2004; Takai and Shigehara, 2004; Jaeger and Marivaux, 2005; Kay, 2005; Seiffert et al., 2005; Marivaux, 2006; Gebo et al., 2007; Gunnell and Ciochon, 2008; Beard et al., 2009; Zollikofer et al., 2009).
Fossils from the Pondaung Formation, especially the relatively rare postcranial and cranial elements that have sometimes been allocated to large-bodied amphipithecids, have played a disproportionate role in recent debates about anthropoid versus adapiform affinities for amphipithecids (Ciochon et al., 2001; Gunnell et al., 2002; Marivaux et al., 2003, 2008; Takai et al., 2003; Kay et al. 2004b; Beard et al., 2005, 2007; Gunnell and Ciochon, 2008). Ongoing paleontological fieldwork in Myanmar has significantly improved our knowledge of primate diversity in the Pondaung Formation (Beard et al., 2007, 2009). At the same time, this work has greatly enhanced the sample of primate postcranial remains that is known from this rock unit (Marivaux etal., 2003, 2008a,b; Beard et al., 2007). These new discoveries of Pondaung primates have highlighted the problems associated with allocating some of the isolated cranial and postcranial elements to the Amphipithecidae. As a result, several dentally unassociated Pondaung specimens that were previously assigned to Amphipithecidae, including a partial postcranial skeleton [National Museum of Myanmar Primates (NMMP) 20] and two purported cranial fragments (NMMP 19 and NMMP 27), are no longer accepted as pertaining to this group (see Beard et al., 2005, 2007; Marivaux et al., 2008a). This leaves only NMMP 39, an isolated primate talus described by Marivaux et al. (2003), as the sole specimen from the Pondaung Formation that may illuminate the nondental anatomy of amphipithecids. Marivaux et al. (2003) cited multiple anthropoid-like features on the NMMP 39 talus as evidence that amphipithecids are anthropoids rather than adapiforms. Gunnell and Ciochon (2008) recently reassessed this specimen and reached a very different conclusion, suggesting that NMMP 39 more closely resembles adapiforms than anthropoids.
During our 2008 field expedition in the Pondaung Formation, we visited the vast outcrops located near Mogaung in central Myanmar (see Fig. 1). Intensive survey of the variegated clays at Thandaung Kyitchaung yielded a second well-preserved fossil primate talus (NMMP 82), which is appropriate in size to pertain to a large-bodied amphipithecid. This specimen is interesting because it hails from the same locality that produced the holotype lower jaw fragment of “Amphipithecus” mogaungensis,1 which was discovered by the well-known paleontologist Barnum Brown in 1923. Like NMMP 39, the new talus was not directly associated with other primate postcranial or dental remains in the field. Here, we describe the morphology of this new talus and compare it with those of other living and fossil primates. We emphasize those talar features that have proven to be useful in reconstructing higher level primate phylogeny (e.g., Gebo, 1986, 1988; Beard et al., 1988; Dagosto and Gebo, 1994) and discuss the osteological features that reflect functional attributes related to locomotor behavior. Most of the metric features for describing and comparing talar anatomy among primates follow the works of Gebo (1988), Gebo et al. (1991, 2001), and Seiffert and Simons (2001). These are illustrated in Figure 2. The specimen is housed in the paleontological collections of the National Museum of the Union of Myanmar in Yangon.
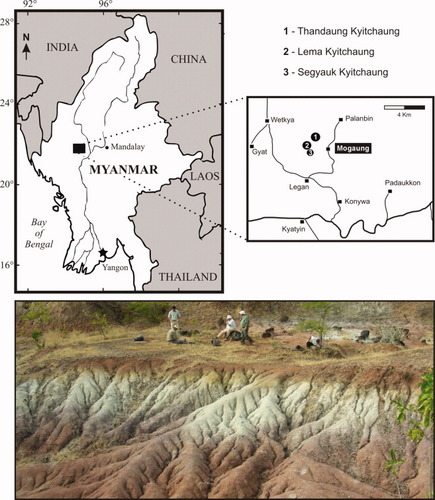
Location map of the fossiliferous locality of Thandaung Kyitchaung (n°1) about 2 km north of the Mogaung village in central Myanmar. The photograph shows the typical badlands of variegated clays from Thandaung Kyitchaung (Picture by Laurent Marivaux). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
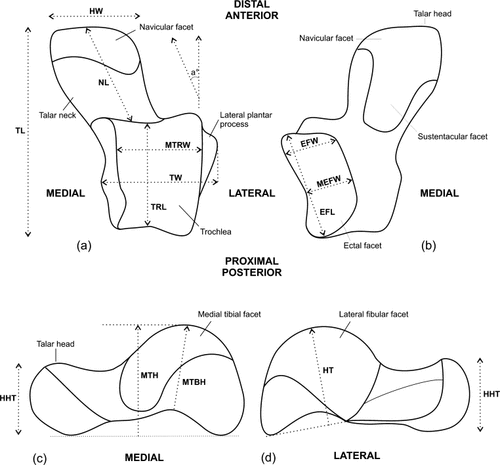
Features and measurements of the talus discussed in this article (modified after Gebo, 1988; Gebo et al., 1991, 2001; Seiffert and Simons, 2001). (a) dorsal view, (b) plantar view, (c) medial view, and (d) lateral view. Metric talar features: TL, talar length; NL, talar neck length; TRL, trochlear length; MTRW, midtrochlear width; TW, talar width (distance from the most lateral point on the fibular facet [laterally projecting talar process] to the most medial point on the tibial facet); MTH, medial talar height (perpendicular distance from the most dorsal aspect of the medial trochlear margin to a chord connecting the most plantar point on the medial talar body to the plantar aspect of the talar head); MTBH, medial talar body height; HT, lateral talar body height (perpendicular distance from the most dorsal point on the lateral trochlea margin to the chord defining the most plantar extent of the anterior and posterior aspects of the ectal facet); HW, talar head width (maximum mediolateral width); HHT, talar head height (maximum dorsoplantar height); EFL, maximum ectal facet length; EFW, maximum ectal facet width; MEFW, minimum ectal facet width; a°, talar neck angle (Tneckangle: medial deviation of the talar neck relative to the anteroposterior axis of the trochlea).
DESCRIPTIONS AND COMPARISONS
NMMP 82 is a well-preserved right talus lacking any apparent postmortem distortion (see Fig. 3). Medially, the specimen suffers from minor abrasion (Fig. 3b), most notably on the talar head, the dorsal part of the talar body, and the most posteroplantar extremity of the bone, where the apex of the medial tubercle is lacking. In overall proportions, NMMP 82 is rather wide relative to its length (Table 1) and moderately tall in medial view. The length of the talar neck plus head is about half of total talar length and barely longer than the length of the trochlea (Table 1), as is generally observed in South American platyrrhine monkeys, notably the medium-sized cebids and most Paleogene omomyids (Table 2). The neck is nearly straight, being only slightly deflected medially (25°) relative to the anteroposterior axis of the trochlea (Fig. 3a). This contrasts with the moderately to very medially angled talar neck observed in practically all platyrrhines (e.g., Gebo et al., 1990, 2001) and basal anthropoids such as Eosimias (Gebo et al., 2000), parapithecids, and propliopithecids (e.g., Gebo et al., 1994; Fleagle and Simons, 1995; Seiffert and Simons, 2001). However, this neck angle can be observed in several modern strepsirhine families such as indriids, cheirogaleids, daubentoniids, and lorisids and in some extinct adapiforms (e.g., Adapis, Leptadapis, and Notharctus). In its low degree of medial deviation, the talar neck of NMMP 82 resembles those of Tarsius and some omomyid taxa as well as many strepsirhines, notably lemurids and galagids (Table 2).
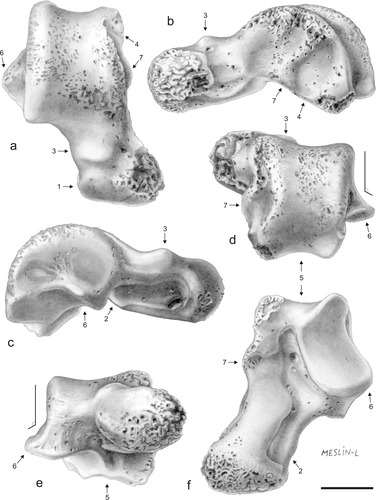
Drawings of NMMP 82, right talus, in dorsal (a), medial (b), lateral (c), posterior [proximal] (d), anterior [distal] (e), and plantar (f) views. Arrows and associated numbers denote anatomical features discussed in the text. Scale bar, 5 mm. Original art by Laurence Meslin, copyright CNRS-Meslin.
| NMMP 82 | NMMP 39 | |
|---|---|---|
| TL | 19.86 | 16.21 |
| NL | 11.7 | 9.2 |
| TRL | 11.55 | 8.8 |
| MTRW | 9.8 | 7.98 |
| TW | 12.85 | 10.56 |
| MTH | 10.54 | 8.45 |
| MTBH | 7.7 | 6.43 |
| HT | 8.63 | 7.66 |
| HW | 8.33 | 8.46 |
| HHT | 6.33 | 6.17 |
| EFL | 10.5 | 8.46 |
| EFW | 5.8 | 5.2 |
| MEFW | 5.16 | 4.4 |
| Tneckangle (a°) | 25° | 28° |
- The measurements taken are illustrated and explained in Figure 2. For NMMP 39, the measurements are from a cast. All measurements were taken to the nearest 0.01 mm using a Stainless digital caliper.
| Taxa | N | NL/TL | NL/TRL | NL/MTRW | HT/MTRW | HT/TRL | MTRW/TRL | HW/HHT | HW/MTRW | TW/TL | ao |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Omomyids | |||||||||||
| Teilhardina belgica | 1 | 56 | 115 | 164 | 150 | 105 | 70 | 138 | 128 | 56 | 25 |
| Tetonius homunculus | 3 | 55 (54–57) | 106 (100–110) | 144 (127–165) | 116 (115–118) | 99 | 79 (67–79) | 140 | 90 | 57 (55–60) | 30 |
| Absarokius abbotti | 1 | 54 | 103 | 150 | 118 | 70 | 67 | 122 | 100 | 51 | 28 |
| Shoshonius cooperi | 3 | 52 (50–55) | 107 (103–112) | 142 (134–155) | 115 (113–116) | 89 (89–90) | 76 (72–79) | 115 (102–138) | 98 (93–105) | 51 (50-51) | 30 |
| ?Omomys | 8 | 51 (49–54) | 101 (96–105) | 123 (115–135) | 107 (98–119) | 89 (84–97) | 82 (74–91) | 124 (118–135) | 95 (89–105) | 56 (55–61) | 25 |
| ?Hemiacodon gracilis | 10 | 51 (48–57) | 99 (93–111) | 126 (104–146) | 110 (96–118) | 86 (77 92) | 75 (67–82) | 126 (116–124) | 94 (78–110) | 57 (53–61) | 25 |
| Washakius insignis | 1 | 52 | 90 | 135 | 118 | 78 | 66 | 106 | 100 | 52 | 30 |
| Arapaphovius gazini | 1 | 58 | 98 | 135 | 126 | 88 | 78 | 112 | 87 | 47 | 26 |
| Necrolemur zitteli | 1 | 52 | 72 | 124 | 113 | 70 | 62 | 103 | 89 | 54 | 30 |
| Microchoerus erinaceus | 1 | 47 | 82 | 123 | 150 | 100 | 66 | 107 | 93 | 51 | |
| Tarsiids | |||||||||||
| Tarsius syrichta | 9 | 49 (40–61) | 87 (72–121) | 99 (84–118) | 76 (67–87) | 68 (55–80) | 89 (71–104) | 118 (114–124) | 81 (76–84) | 52 (44–56) | 17 |
| Tarsius bancanus | 6 | 49 (45–54) | 89 (76–105) | 98 (84–118) | 80 (68–96) | 73 (60–86) | 92 (84–111) | 124 (123–125) | 87 (82–92) | 51 (46–58) | 18 |
| SH-Tarsiid V11854 | 1 | 47 | 87 | 107 | 97 | 79 | 82 | 127 | 89 | 58 | 22 |
| Platyrrhines | |||||||||||
| Dolichicebus gaimanensis | 1 | 54 | 94 | 125 | 109 | 82 | 75 | 65 | 32 | ||
| IGM-KU 8803 | 1 | 60 | 104 | 141 | 108 | 79 | 73 | 84 | 91 | 64 | 35 |
| IGM-KU 8802 | 1 | 59 | 90 | 132 | 103 | 76 | 74 | 106 | 109 | 68 | 32 |
| Cebupithecia sarmientoi | 1 | 54 | 105 | 136 | 105 | 81 | 77 | 110 | 115 | 59 | 35 |
| Cebuella pygmaeus | 7 | 63 (55–69) | 114 (94–124) | 125 (109–135) | 87 (81–91) | 79 (70–86) | 91 (82–95) | 121 (112–136) | 86 (82–91) | 61 (59–64) | 42 |
| Callithrix jacchus | 10 | 66 (60–72) | 125 (112–143) | 129 (112–145) | 89 (82–95) | 86 (82–93) | 97 (91–104) | 124 (11–136) | 88 (83–95) | 65 (58–74) | 40 |
| Callithrix argentala | 6 | 64 (59–67) | 124 (114–132) | 134 (129–141) | 88 (83–92) | 81 (78–85) | 92 (87–102) | 131 (119–140) | 94 (87–101) | 61 (59–63) | 38 |
| Saguinus leucopus | 3 | 59 (59–60) | 116 (103–120) | 154 (149–157) | 108 (103–111) | 72 (71–72) | 82 (81–85) | 120 (118–124) | 110 (108–111) | 62 (58–65) | 37 |
| Saguinus midas | 4 | 60 (57–63) | 111 (105–126) | 138 (131–150) | 100 (95–108) | 74 (67–77) | 84 (82–84) | 117 (114–120) | 101 (98–105) | 66 (63–69) | 34 |
| Saguinus oedipus | 20 | 59 (51–64) | 106 (100–122) | 121 (102–132) | 91 (78–101) | 76 (67–84) | 88 (80–93) | 118 (111–127) | 88 (80–95) | 65 (60–71) | 37 |
| Callimico goeldi | 10 | 60 (54–66) | 111 (101–130) | 125 (110–141) | 85 (81–89) | 76 (70–82) | 89 (82–94) | 125 (119–142) | 92 (87–96) | 69 (60–75) | 38 |
| Saimiri sciureus | 10 | 54 (48–61) | 99 (87–103) | 133 (112–150) | 108 (100–117) | 76 (69–86) | 85 (80–90) | 130 (124–137) | 98 (91–120) | 58 (53–64) | 30 |
| Cebus apella | 6 | 57 (54–61) | 97 (94–105) | 118 (114–122) | 104 (100–106) | 86 (77–91) | 82 (77–86) | 122 (116–133) | 94 (92–98) | 66 (61–71) | 32 |
| Cebus capucinus | 5 | 56 (53–62) | 101 (95–103) | 146 (139–159) | 134 (127–143) | 90 (86–97) | 67 (60–71) | 127 (118–138) | 123 (119–132) | 70 (68–73) | 33 |
| Callicebus torquatus | 2 | 52 (52–53) | 91 (89–94) | 144 | 118 (117–119) | 82 (80–84) | 77 (76–77) | 131 (125–136) | 118 (115–121) | 56 (55–58) | 34 |
| Callicebus donaphilus | 3 | 51 (49–53) | 85 (83–87) | 125 (110–138) | 112 (103–121) | 83 (73–94) | 77 (68–84) | 142 (140–146) | 102 (100–104) | 59 (58–61) | 30 |
| Aotus azarae | 6 | 53 (53–55) | 96 (92–100) | 144 (131–153) | 119 (112–126) | 77 (73–81) | 83 (77–86) | 131 (125–139) | 110 (99–121) | 54 (51–56) | 31 |
| Pithecia pithecia | 6 | 52 (50–56) | 86 (81–91) | 132 (123–141) | 105 (95–119) | 68 (66–71) | 65 (60–70) | 135 (129–143) | 118 (110–125) | 69 (63–72) | 35 |
| Alouatta seniculus | 5–6 | 50 (47–51) | 85 (81–92) | 116 (98–133) | 93 (85–107) | 68 (63–74) | 74 (63–86) | 134 (117–147) | 100 (91–117) | 70 (65–76) | 33 |
| Ateles belzebuth | 1 | 45 | 77 | 87 | 95 | 84 | 88 | 104 | 81 | 68 | 35 |
| Adapiforms | |||||||||||
| Cantius ralstoni | 4 | 120 (112–129) | 74 (69–81) | 62 (54–67) | 15 | ||||||
| Cantius trigonodus | 3 | 46 (41–50) | 81 (71–88) | 138 (129–148) | 130 (116–141) | 82 (82–82) | 58 (55–62) | 129 (125–136) | 123 (118–127) | 50 | 14 |
| Cantius abditus | 2 | 40 (40–41) | 77 (65–89) | 131 (124–139) | 132 (128–137) | 66 (61–72) | 55 (48–64) | 128 | 59 (58–59) | 26 | |
| Notharctus tenebrosus | 4 | 46 (42–51) | 86 (79–91) | 146 (126–164) | 152 (143–161) | 85 (82–88) | 59 (–63) | 121 (119–122) | 128 (125–132) | 59 (56–62) | 35 |
| Notharctus pugnax | 10 | 43 (40–45) | 82 (75–93) | 139 (119–161) | 158 (138–185) | 93 (85–110) | 58 (45–66) | 123 (122–125) | 128 (109–139) | 57 (53–63) | 29 |
| Smilodectes gracilis | 7 | 43 (41–46) | 72 (67–77) | 136 (120–163) | 149 (133–167) | 78 (69–84) | 53 (47–60) | 130 (126–133) | 130 (120–142) | 59 (55–62) | 30 |
| Adapis parisiensis | 2 | 33 (32–33) | 50 (50–51) | 80 (78–84) | 117 (114–121) | 73 (72–75) | 62 (59–65) | 127 | 116 | 67 ((63–72) | 38 |
| Leptadapis magnus | 5 | 37 (31–43) | 60 (43–75) | 91 (83–101) | 124 (100–150) | 80 (68–99) | 66 (52–79) | 132 | 128 | 65 (55–82) | 37 |
| Lemuriforms | |||||||||||
| Eulemur fulvus | 17–25 | 46 (41–50) | 85 (75–111) | 128 (116–149) | 119 (110–132) | 75 (68–83) | 66 (55–76) | 121 (110–137) | 111 (98–129) | 63 (54–66) | 31 (25–40) |
| Lemur catta | 8–13 | 47 (43–51) | 90 (76–99) | 131 (109–149) | 119 (109–149) | 81 (73–86) | 69 (61–76) | 125 (120–139) | 112 (97–124) | 69 (58–92) | 33 (20–42) |
| Varecia variegata | 11–14 | 48 (46–51) | 89 (79–98) | 135 (120–154) | 130 (119–144) | 83 (74–91) | 66 (54–82) | 129 (125–139) | 122 (102–139) | 68 (59–73) | 36 (24–47) |
| Hapalemur griseus | 11–12 | 46 (41–55) | 86 (74–99) | 127 (102–163) | 119 (104–135) | 80 (73–86) | 68 (57–78) | 131 (126–139) | 112 (126–139) | 61 (57–66) | 29 (26 40) |
| Avahi laniger | 7–11 | 42 (46–72) | 76 (67–85) | 121 (102–162) | 119 (98–147) | 70 (62–76) | 64 (46–72) | 122 (115–129) | 115 (102–140) | 61 (54–67) | 32 (22–40) |
| Propithecus verreauxi | 9–11 | 45 (35–50) | 87 (72–98) | 121 (95–142) | 105 (97–112) | 74 (66–88) | 72 (62–84) | 121 (115–129) | 108 (100–119) | 61 (55–67) | 30 (25–37) |
| Indri Indri | 8–12 | 45 (37–50) | 86 (62–99) | 121 (89–149) | 106 (96–117) | 74 (67–88) | 71 (58–84) | 114 (108–120) | 108 (91–124) | 63 (50–71) | 29 (20–40) |
| Lepilemur sp. | 17 | 48 (43–53) | 100 (80–134) | 133 (114–160) | 113 (104–129) | 85 (74–115) | 75 (62–89) | 137 (129–147) | 113 (95–164) | 59 (53–64) | 21 (12–30) |
| Daubentonia madagascariensis | 1–5 | 44 (38–52) | 81 (73–90) | 126 (100–171) | 142 (141–143) | 74 (73–74) | 67 (51–90) | 140 | 145 | 58 (51–66) | 38 (30–41) |
| Cheirogaleus medius | 1–6 | 49 (46–55) | 88 (81–97) | 122 (116–131) | 101 (97–104 | 70 (66–71) | 72 (67–78) | 126 | 90 | 58 (51–64) | 27 (25–30) |
| Microcebus sp (murinus) | 14–15 | 49 (41–56) | 110 (96–126) | 147 (125–171) | 114 (100–135) | 85 (75–96) | 75 (68–91) | 127 (121–140) | 106 (95–120) | 49 (42–60) | 20 (14–25) |
| phaner furcifer | 1 | 47 | 103 | 128 | 124 | 100 | 80 | 119 | 100 | 46 | 38 |
| Lorisiforms | |||||||||||
| Galago senegalensis | 1–6 | 52 (44–57) | 105 (93–114) | 134 (115–150) | 123 | 84 | 79 (68–88) | 81 | 97 | 39 (34–44) | 23 (15–30) |
| Galagoides demidovii | 6–13 | 50 (44–55) | 97 (90–107 | 129 (116–155) | 105 (95–119) | 81 (74–88) | 75 (62–84) | 118 (108–125) | 99 (86–108) | 39 (34–43) | 25 (15–30) |
| Otolemur crassicaudalus | 4–7 | 53 (48–59) | 95 (86–104) | 150 (128–193) | 130 (113–152) | 80 (76–83) | 64 (52–75) | 120 (116–126) | 115 (104–131) | 35 30–42) | 27 (23–30) |
| Euoticus elegantulus | 2–16 | 47 (39–51) | 91 (76–106) | 128 (111–153) | 112 (109–115) | 83 (78–88) | 71 (61–80) | 117 (110–125) | 101 (95–106) | 36 (30–41) | 24 (16–30) |
| Perodicticus potto | 7–15 | 54 (48–62) | 85 (73–100) | 135 (121–151) | 116 (100–141) | 72 (64–87) | 63 (54–62) | 167 (153–188) | 134 (111–172) | 69 (59–82) | 40 (27–52) |
| Arctocebus calabarensis | 2–11 | 42 (35–50) | 68 (54–81) | 110 (96–150) | 152 (126–191 | 95 (86–105) | 63 (54–75) | 151 (148–154) | 118 (109–129) | 66 (59–70) | 33 (27–45) |
| Nycticebus coucang | 4–13 | 51 (40–62) | 79 (59–95) | 130 (105–157 | 114 (103–132) | 67 (54–80) | 60 (53–67) | 181 (170–193 | 143 (137–154 | 67 (56–82) | 40 (26–55) |
| Shanghuang | |||||||||||
| SH ProtoAnth. V12305 | 1 | 49 | 142 | 102 | 117 | 84 | 72 | 138 | 111 | 56 | 35 |
| SH-ProtoAnlh. V12306 | 1 | 49 | 120 | 95 | 103 | 81 | 79 | 135 | 96 | 54 | 33 |
| SH-Eosimiids V11846 | 1 | 51 | 130 | 100 | 112 | 86 | 77 | 136 | 106 | 52 | 27 |
| SH-Eosimiids V11849 | 1 | 51 | 133 | 101 | 96 | 73 | 76 | 134 | 103 | 48 | 30 |
| SH-Eosimiids V11855 | 1 | 48 | 130 | 92 | 126 | 89 | 71 | 134 | 104 | 54 | 29 |
| SH-Eosimiids V12301 | 1 | 129 | 78 | 60 | 27 | ||||||
| SH-Eosimiids V12302 | 1 | 124 | 104 | 132 | 101 | 24 | |||||
| SH-Eosimiids V12303 | 1 | 48 | 122 | 92 | 118 | 88 | 75 | 159 | 96 | 52 | 30 |
| SH-Eosimiids V12304 | 1 | 113 | 76 | 97 | 21 | ||||||
| SH-Haplorhines V11857 | 1 | 54 | 104 | 75 | 135 | 51 | 24 | ||||
| SH-Haplorhines V12297 | 1 | 119 | 100 | 84 | 18 | ||||||
| SH-Haplorhines V12298 | 1 | 52 | 125 | 149 | 111 | 93 | 84 | 149 | 107 | 49 | 16 |
| SH-Haplorhines V12299 | 1 | 51 | 117 | 152 | 99 | 76 | 77 | 138 | 110 | 45 | 18 |
| Pondaung | |||||||||||
| NMMP 82 | 1 | 57 | 105 | 115 | 96 | 87 | 91 | 137 | 106 | 65 | 28 |
| NMMP 39 | 1 | 59 | 101 | 119 | 88 | 75 | 85 | 132 | 85 | 65 | 25 |
Despite the slight distomedial abrasion of the talus, it remains clear that the head expands more medially than laterally, a condition that also occurs in NMMP 39 (see Fig. 4). In anterior view (Fig. 3e), the head appears ovoid in shape, being wider than high, with its long axis oriented mediolaterally relative to the dorsal plane of the trochlear surface. In dorsal view (Fig. 3a), the distal edge of the head, which articulates with the navicular, is not rounded as it is in NMMP 39 (Fig. 4b). Instead, this joint surface is mediolaterally straight and dorsoventrally steep sided, a condition that is unusual among primates (except for Tarsius). The navicular facet is very limited on the lateral aspect of the head (Fig. 3, arrow 1). In contrast, it expands far onto its dorsal aspect but not uniformly, being less developed dorsomedially than dorsolaterally. Plantarly, this facet is confluent with the sustentacular facet of the talar neck (Fig. 3f). The sustentacular facet is not strictly plantar in position, because it appears to face slightly medially (obliquely). However, the sustentacular facet does not extend onto the medial margin of the neck (strongly oblique) as it does in a few primates (e.g., Varecia, Daubentonia, Galago, Otolemur, Saimiri, Callicebus, and Cebus). The lateral aspect of the neck is widely and deeply excavated, marking the site for attachment of the anterior talocalcaneal ligament (Fig. 3, arrow 2). Dorsolaterally (Fig. 3a–e), the neck bears a distinct bony protuberance (Fig. 3a–e, arrow 3) that probably functioned to limit dorsiflexion at the talocrural joint. This dorsal tibial stop facet on the talar neck is common among primates, notably in many platyrrhines (e.g., Saimiri sciureus, Alouatta, Ateles, and Pithecia), several strepsirhines (e.g., Lepilemur, Propithecus, Eulemur, and Otolemur), and adapiforms (e.g., Notharctus).
In anterior view (Fig. 3e), the trochlea is only moderately grooved, displaying rounded trochlear rims. The latter structures are asymmetrical with the medial rim being slightly more elevated dorsally. The medial rim extends slightly farther distally onto the talar neck than the lateral rim. The medial and lateral sides of the trochlea are steep, a feature that has been described as characteristic of anthropoids, Tarsius, and omomyids (Gebo, 1986; Beard et al., 1988; Dagosto and Gebo, 1994; Gebo et al., 2000). In dorsal view (Fig. 3a), the medial and lateral trochlear rims diverge slightly distally, yielding a faint wedge-like shape to the trochlear surface. Posteriorly, the medial trochlear margin becomes less distinct and the trochlear surface narrows medially but widens again proximally. This shallow notch on the medial trochlear rim (also present in NMMP 39; Fig. 4) is generated by a strong attachment for the posterior talotibial part of the deltoid ligament on the posteromedial aspect of the talus (Fig. 3a,b, arrow 4). The lateral rim of the trochlea differs in being long, straight, and continuous (Fig. 3a) showing a regular arc of curvature (Fig. 3c). This condition contrasts with that observed in all strepsirhines, where the posterior lateral tibiotalar joint region is modified by the passage of the flexor hallucis longus tendon, which generates a groove posterolaterally. In NMMP 82 (Fig. 3d–f, arrow 5), this groove is plantad and in a midline position relative to the posterior trochlear facet, as is also the case in NMMP 39, Tarsius, omomyids, and all anthropoids (Gebo, 1986; Beard et al., 1988; Dagosto and Gebo, 1994; Gebo et al., 2000). NMMP 82 lacks a prominent projecting posterior trochlear shelf, thereby resembling the condition in many extinct and extant anthropoids as well as in NMMP 39. In contrast, a prominent trochlear shelf characterizes most extant strepsirhines and Paleogene adapiforms, especially notharctids in which it is particularly strongly developed. Omomyids and eosimiids possess a less-developed shelf (e.g., Szalay, 1974; Godinot and Dagosto, 1983; Gebo, 1988; Covert and Williams, 1994; Dagosto and Gebo, 1994; Gebo et al., 2001). In NMMP 82, there is no apparent discontinuity between the groove of the trochlea and the groove for the flexor muscle in contrast to those anthropoid taxa that bear a very small posterior trochlear shelf (e.g., Apidium, Proteopithecus, Aegyptopithecus, Cebus, Pithecia, Alouatta, and some cercopithecoids). Such a discontinuity is often absent in other platyrrhines, particularly callitrichids (Dagosto etal., 2010). In NMMP 82, the groove for the tendon of the flexor muscle is posteroplantarly buttressed by small medial and lateral tubercles, the medial of which appears relatively larger despite the breakage noted earlier (Fig. 3d). The lateral tubercle is small, forming part of the posterolateral edge of the ectal facet. This plantar posterior articulation with the calcaneus is wide and uniformly concave but its lateral margin is slightly indented near its midpoint.
In NMMP 82, the lateral talar facet, which articulates with the malleolus of the fibula, is entirely vertical (Fig. 3d,e) rather than sloping gently laterally over its entire extent as is the case in all living strepsirhines and those adapiforms for which the talus is known. A steep and straight-sided lateral wall of the trochlea is characteristic of anthropoids, Tarsius, and omomyids (Gebo, 1986, 1988; Beard et al., 1988). In NMMP 82, the plantar surface flares abruptly laterally, forming a moderately projecting, triangular talar process (Fig. 3a–f, arrow 6). The surface of this plantar process appears less extended anteroposteriorly and laterally in NMMP 82 compared with its projection in most living strepsirhines (except galagids and cheirogaleids) and adapiforms and in some cercopithecoids (e.g., Macaca and Cercopithecus). NMMP 82 resembles small platyrrhines in this feature (Table 2).
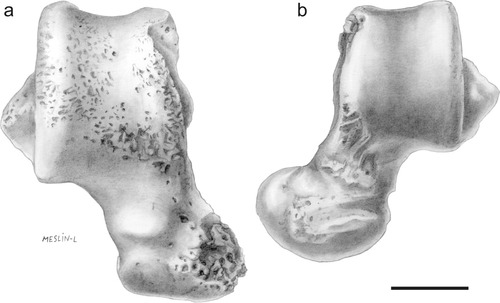
The medial edge of NMMP 82 appears moderately elevated (Fig. 3b) with a dorsoplantarly robust and tall talar body (Table 1). The medial talar facet, which articulates with the malleolus of the tibia, is distinct morphologically from the condition in many strepsirhines or anthropoids. In NMMP 82, this malleolar facet is obliquely oriented and rather limited, being fairly deep (dorsoplantarly) but plantarly short (anteroposteriorly) in its distal aspect, and shallow (dorsally restricted) in its proximal aspect (see Fig. 5). As such, it differs substantially from the “full” condition (sensu Gebo, 1986, 1988)—that is, extensive (deep and large)—found in strepsirhines, adapiforms, Tarsius, and omomyids. Although proximally reduced as in anthropoids (e.g., Gebo et al., 2000), the medial malleolar facet in NMMP 82 also differs from anthropoid tali in being distally deep and not so elevated from the plantar edge of the talar body (see Fig. 5). Furthermore, it neither extends far distally onto the medial side of the talar neck nor does it curves medially as is the case in most strepsirhines and anthropoids. The medial talotibial facet in NMMP 82 differs from the derived condition found in most catarrhines, characterized by exaggerated medial and plantar flaring that forms the deep “cup-like” stop for the malleolus (cotylar fossa) (e.g., Gebo and Simons, 1987; Dagosto, 1990; Fleagle and Simons, 1995; Seiffert and Simons, 2001). Posteriorly, the medial aspect of NMMP 82 is also marked by a deep and extensive surface for attachment of the posterior talotibial part of the deltoid ligament, which extends dorsally over the medial trochlear rim in generating the shallow notch discussed previously (Fig. 3a,b, arrow 4). Just in front of this posterior surface and just behind the talotibial facet, the talar body extends outward plantarly forming a distinct protuberance (Fig. 3b,d,f, arrow 7). Similar medial bony protuberances have been noticed on the tali of some platyrrhines (e.g., Aotus, Callicebus, Saimiri, Cebus, IGM-KU 8802; Gebo et al., 1990) and eosimiids (Gebo et al., 2001), but such structures are rarely found on tali of omomyids and are always absent in adapiforms and strepsirhines (Gebo et al., 2001). These protuberances show different degrees of development among various anthropoid taxa but differ from the condition found in NMMP 82 in being more posteriorly placed.
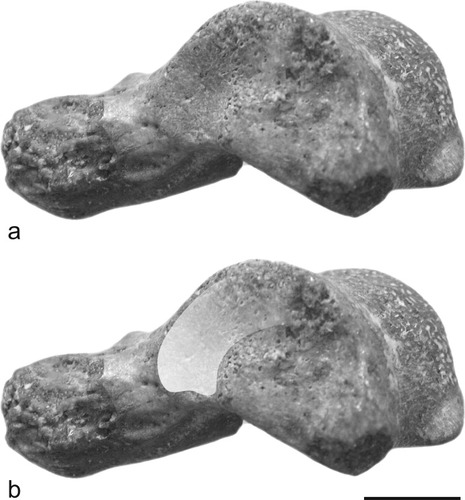
Photographs of the NMMP 82 talus in medial views (a and b). Note the medial talar facet articulating with the malleolus of the tibia. Shading (b) indicates extent of the medial facet, which is obliquely oriented and rather limited, being fairly deep (dorsoplantarly) but plantarly short (anteroposteriorly) in its distal aspect and shallow (dorsally restricted) in its proximal aspect. Scale bar, 5 mm. Original photographs by Laurent Marivaux.
DISCUSSION
Phylogenetic affinities
The NMMP 82 talus from Thandaung Kyitchaung was not found in association with other primate postcranial or dental remains. Therefore, any assessment of its phylogenetic affinities rests solely on its morphology. Despite the clear difference in size between NMMP 82 and NMMP 39 (NMMP 82 being ∼20% larger; Fig. 4), these two tarsal elements are comparable in terms of their overall structure except for the talar head, which is more rounded distally in NMMP 39 (see Fig. 4).
NMMP 82 and NMMP 39 display a suite of morphological characteristics otherwise found only in haplorhine primates. These features include: mid-trochlear position of the groove for the flexor hallucis longus muscle, steep talofibular facet, absence of posterior trochlear shelf, and a long talar neck in relation to trochlear length (Table 3). As such, these two Pondaung tali differ substantially from those of living strepsirhine and fossil adapiform primates, all of which show divergent talar features (i.e., lateral position on the posterior trochlea of the groove for the flexor hallucis longus muscle, sloping talofibular facet, strong and projecting posterior trochlear shelf, and relatively short talar neck; Table 3). NMMP 82 does not exhibit the “full” facet condition regarding the shape of the medial talotibal facet, which is characteristic of “prosimian” primates (living strepsirhines, adapiforms, Tarsius, and omomyids). In contrast, NMMP 82 displays a reduced and obliquely oriented talotibial facet resembling anthropoid tali in this regard. However, in NMMP 82, this facet is not as shallow as in our comparative sample of anthropoids. In fact, the configuration of the talotibial facet in NMMP 82 is quite unusual and appears to be autapomorphic in some respects (e.g., absence of a “cup-like” tibial stop extending onto the talar neck). In sum, among the talar features that have been used to reconstruct higher-level primate phylogeny, it must be emphasized that character states found in NMMP 82 resemble those of haplorhine primates, notably anthropoids, and differ dramatically from alternative conditions found in living strepsirhines and fossil adapiforms.
| Talar characters | Position of the Flexor fubularis groove/ trochlea axis | Shape of the lateral fibular facet | Projection of the lateral talar process | Shape and size of the medial tibial facet | Posterior trochlear shelf | Talar neck length |
|---|---|---|---|---|---|---|
| Strepsirhines | Lateral | Sloped | Strongly extended anteroposteriorly and laterally (except in galagines and cheirogaleids) | Extensive (deep and large) = “full” | Large, prominent | Short (moderate in lorisiforms) |
| Adapiforms | Lateral | Sloped | Strongly extended | Full | Large, prominent (very prominent in notharctids) | Short |
| Omomyiforms | Central | Steep sided | Slight | Full | Large/small | Moderately long |
| Tarsius | Central | Steep sided | Slight | Full | None/small | Moderately long |
| Eosimiids | Central | Steep sided | Slight | Limited, elevated from the plantar edge | Small | Moderately long |
| Anthropoids (platyrrhines) | Central | Steep sided | Slight | Limited, elevated from the plantar edge | None/small | Long |
| NMMP 82 | Central | Steep sided | Slight | Limited but deep | None | Long |
NMMP 82 augments the meager sample of postcranial remains documenting large-bodied primates from the Pondaung Formation, which otherwise consists of only two specimens: the partial postcranial skeleton (NMMP 20) described by Ciochon et al. (2001) and Marivaux et al. (2008a) and the isolated talus (NMMP 39) described by Marivaux et al. (2003). Despite the small sample of large-bodied primate postcranial remains currently known from the Pondaung Formation, taxonomic and phylogenetic interpretations of this sample have diverged radically. Virtually all authorities agree that the NMMP 20 partial postcranial skeleton pertains to an adapiform, although there is no current consensus as to whether this specimen should be allocated to the Amphipithecidae (the higher level relationships of which remain contested) as opposed to some undoubted adapiform clade, such as Sivaladapidae (Ciochon et al., 2001; Beard et al., 2007; Gebo et al., 2007; Marivaux et al., 2008a). Likewise, although Marivaux et al. (2003) interpreted the NMMP 39 talus as showing clear anthropoid affinities, Gunnell and Ciochon (2008) regarded the same specimen as adapiform-like, potentially rendering it compatible with the NMMP 20 partial skeleton (which lacks a talus).
These conflicting interpretations of the affinities of the NMMP 39 talus give rise to very different opinions about the number of large-bodied primate taxa that is documented by postcranial remains from the Pondaung Formation. That is, if Gunnell and Ciochon's (2008) assessment of the NMMP 39 talus is accurate, then all of the large-bodied primate postcranial remains from the Pondaung Formation could conceivably pertain to a single primate taxon (or at least a single higher level primate taxon, such as Amphipithecidae). On the other hand, if the NMMP 39 talus is that of an anthropoid as Marivaux et al. (2003) have argued, then at least two higher level primate taxa (Adapiformes and Anthropoidea) are represented by the Pondaung sample of large-bodied primate postcranials. As noted earlier, the new talus being described here (NMMP 82) is larger but morphologically close to the NMMP 39 talus originally described by Marivaux et al. (2003) and reassessed by Gunnell and Ciochon (2008). As such, the ongoing controversy regarding the affinities of the large-bodied primate postcranial sample from the Pondaung Formation hinges on whether the entire sample might pertain to a single higher level taxon (Gunnell and Ciochon, 2008) or whether a fundamental morphological and taxonomic discrepancy segregates NMMP 20 from NMMP 39 and NMMP 82 (Marivaux et al., 2003, 2008a; Beard et al., 2007).
A novel way of assessing whether the NMMP 82 talus and the NMMP 20 partial skeleton might pertain to the same taxon is by investigating the morphological compatibility of the subtalar joint, complementary aspects of which are preserved in these two specimens. If NMMP 82 and NMMP 20 pertain to the same taxon, we would expect the subtalar joint surface of the NMMP 82 talus to conform with its counterpart on the NMMP 20 calcaneus. Alternatively, if these subtalar joint surfaces fail to correspond morphologically, then we can infer that the NMMP 82 talus and the NMMP 20 calcaneus should be allocated to different taxa that happen to be similar in size. Our analysis of subtalar joint compatibility in these specimens reveals a substantial functional mismatch between these two tarsal bones (see Fig. 6). For instance, the angle of curvature of the ectal facet of the talus does not correspond well with that of the posterior calcaneal facet (see Fig. 6). Similarly, the sustentacular facet of the talus fails to complement the anterior calcaneal facet (see Fig. 6). This mismatch cannot be overcome simply by a change in size (i.e., a talus from a smaller or larger individual); it is a matter of shape. The NMMP 82 talus appears to be too short and too broad with respect to the overall proportions of the NMMP 20 calcaneus (see Fig. 6). A narrower and longer talus than NMMP 82 would be far more compatible with the NMMP 20 calcaneus.
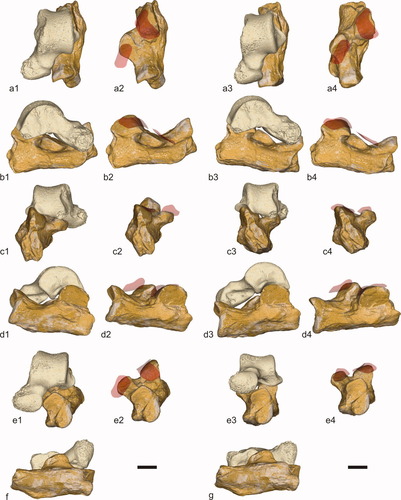
Three-dimensional (3D) rendering of the subtalar joint formed by a chimera associating the NMMP 20 calcaneus and the NMMP 82 talus (reversed). The images of the NMMP 82 talus and the NMMP 20 calcaneus have been generated from 3D data obtained by X-ray microtomography (Microtomograph VISCOM [University of Poitiers] and Synchrotron [ESRF, Grenoble], respectively): dorsal (a), medial (b), proximal (c), lateral (d), distal (e), and plantar (f–g) views. The articular surfaces of the talus (anterior sustentacular facet and posterior ectal facet) have been digitally rendered and placed on the corresponding calcaneal facets to analyze the degree of compatibility of the subtalar joint in eversion (1–2) and in inversion (3–4). Scale bar, 5 mm.
The mismatch is also evident in the transverse tarsal joint. One of the more interesting features of the NMMP 20 calcaneus is the relatively vertical inclination of the major axis of its cuboid facet, which lies ∼55–60° to the horizontal (Fig. 6e), a value most comparable to that observed on calcanei of lorises among living primates (Ciochon et al., 2001) and the Pondaung sivaladapid Kyitchaungia among fossil adapiforms (Beard et al., 2007: Fig. 3e). Primates with such vertically oriented cuboid facets (e.g., lorisines, galagines, adapines, and Megaladapis) have talar heads that are dorsomedially oriented, while those with lower cuboid angles (e.g., indriids, lemurids, and anthropoids) have dorsolaterally oriented talar heads (Dagosto, 1986; Wunderlich et al., 1996). The NMMP 82 talus has a slightly dorsolaterally oriented talar head (Fig. 6e). Given the apparent relationship between the torsion angle of the talar head and the angle of orientation of the cuboid facet (see Fig. 7), a hypothetical NMMP 82/NMMP 20 combination would be unique among nonhuman primates, yielding impractical functional consequences for a grasping arboreal quadruped. When the subtalar joint is in eversion (Fig. 6e1), such an arrangement results in a more acute angle between the cuboid and navicular than the more normal horizontal alignment typical of primates (Decker and Szalay, 1974). In subtalar inversion (Fig. 6e3), the cuboid and navicular end up more vertically aligned and at a more acute angle to each other than is typical in nonhuman primates (Decker and Szalay, 1974). Without invoking significant compensation elsewhere in the midtarsal region, this would result in a mediolaterally arched midfoot with restricted subtalar motion, functionally (though not morphologically) analogous to the midtarsal restraining mechanism of the human foot (Elftman, 1960; Kidd et al., 1996). A transverse tarsal joint like this would be unexpected in a primate that would otherwise appear to be capable of a normal degree of subtalar motion, judging by the long and narrow facets of the posterior part of the subtalar joint. As such, it is both morphologically and functionally unlikely that NMMP 82 and NMMP 20 pertain to the same kind of primate.

Talar head torsion angle (X axis) plotted against the angle of the cuboid facet of the calcaneus (Y axis). Positive talar head angles are dorsolaterally oriented; negative values indicatedorsomedial orientation. Open squares are Lemuriformes, closedsquares are Adapiformes, open triangles are extant anthropoids,and closed triangles are fossil anthropoids (Apidium and Aegyptopithecus). The star is the position that would be occupied by a chimera of the NMMP 20 calcaneus and the NMMP 82 talus. Note that no other living or fossil primates occupy this quadrant of the graph.
To summarize our current knowledge of the large- bodied primate postcranial remains from the Pondaung Formation, the three specimens currently available fall into two different groups: one of which consists of the NMMP 20 partial skeleton and the other of which includes the two isolated primate tali, NMMP 39 and NMMP 82. The higher level phylogenetic affinities of NMMP 20 are not currently contested, because all authorities agree that this partial skeleton pertains to some type of large-bodied adapiform (Ciochon et al., 2001; Gunnell and Ciochon, 2008; Marivaux et al., 2008a). Ongoing debate focuses on whether NMMP 20 can be allocated to the Amphipithecidae (Ciochon et al., 2001; Beard et al., 2007; Gunnell and Ciochon, 2008; Marivaux et al., 2008a). In contrast to NMMP 20, NMMP 39 and NMMP 82 show a suite of anatomical features indicating haplorhine and/or anthropoid affinities (Marivaux et al., 2003; also see above). The divergent phylogenetic affinities of NMMP 20 and NMMP 82 are corroborated by the functional incompatibility outlined previously, making it clear that two higher level taxa of large-bodied primates are documented in the Pondaung Formation (Beard et al., 2007; Marivaux et al., 2008a).
Which of these two large-bodied primate taxa documented by postcranial remains is more likely to be allocated to Amphipithecidae, a taxon that to date is definitively known only by jaws and teeth? On the basis of humeral measurements, Ciochon et al. (2001) estimated that NMMP 20 pertained to a primate having a body mass of 5–6 kg. Based on regressions of talar dimensions against body mass in living primates, NMMP 82 belonged to a primate having a body mass ranging from about 3.7–11 kg (estimated2 from lemur-only bivariate regression equations based on several linear talar dimensions provided by Dagosto and Terranova, 1992). Therefore, both of the Pondaung primate taxa documented by postcranial remains are appropriate in size to pertain to a large-bodied amphipithecid such as Pondaungia. Given that neither size nor directly associated cranial and postcranial remains provide a clear answer, the only criterion we have for choosing which of the two large-bodied Pondaung primate taxa documented by postcranial remains is most appropriately allocated to Amphipithecidae is the degree of congruence between postcranial and craniodental data sets that results from these different taxonomic allocations.
In their recent analysis of the phylogeny of early Cenozoic adapiforms and other primates, Seiffert et al. (2009) noted that amphipithecids are most parsimoniously interpreted as anthropoids, regardless of whether the adapiform-like NMMP 20 postcranial skeleton is accepted as pertaining to this group. To be specific, Amphipithecidae is reconstructed as diverging along the stem lineage of anthropoids if NMMP 20 is included as part of the amphipithecid hypodigm, whereas Amphipithecidae is most parsimoniously reconstructed as a crown anthropoid clade (sister group of Platyrrhini) if NMMP 20 is excluded from that group. The latter result agrees with those reported by Beard et al. (2009) and Zollikofer et al. (2009). Apparently, the craniodental evidence supporting the anthropoid affinities of amphipithecids is so compelling that even the addition of adapiform-like postcranial features found in NMMP 20 cannot overturn this phylogenetic result. Accordingly, greater congruence between postcranial and craniodental data sets is achieved by allocating the anthropoid-like tali (NMMP 39 and NMMP 82) to the Amphipithecidae. In other words, there is no logical basis for arguing that the adapiform-like partial skeleton known from the Pondaung Formation pertains to Amphipithecidae when a more parsimonious choice is available.
NMMP 82 belonged to a relatively large-bodied individual of Pondaungia, whereas NMMP 39 belonged to a smaller (or possibly younger) individual. It remains unclear whether these two tali represent sequential growth stages of a monomorphic species, different sexes of a dimorphic species, or two different species of Pondaungia, an issue that is complicated by the possibility of a high level of sexual dimorphism in this group (Jaeger et al., 2004).
Functional interpretation
Although the NMMP 20 partial skeleton was formerly thought to pertain to a large-bodied amphipithecid (i.e., Pondaungia; Ciochon et al., 2001; Kay et al., 2004), this allocation can no longer be sustained in light of the evidence presented here (see also Beard et al., 2007; Gebo et al., 2007; Marivaux et al., 2008a). Accordingly, the skeletal anatomy of amphipithecids is now limited to the NMMP 39 and NMMP 82 tali, rendering these specimens the sole source of information regarding the postural and locomotor behavior of these Eocene primates. Although further postcranial evidence is obviously necessary to understand the positional behavior of Pondaungia more adequately, the new NMMP 82 talus provides a suite of osteological features that offer insights into leg function and locomotion in this genus.
The talus connects the leg to the foot, and as such, it plays a critical role in terms of posture and locomotion because it is responsible for dorsiflexion and plantarflexion of the foot. The fact that NMMP 82 displays tall and steep-sided medial and lateral walls of the trochlea indicates that this ankle was probably restricted primarily to flexion and extension motions in a parasagittal plane (Gebo, 1988; Dagosto, 1990; Fleagle and Simons, 1995). However, the trochlea is not deeply grooved and the trochlear rims are not as sharp as those of specialized leapers, where only one primary plane of movement is needed at the talocrural joint to maximize stability during a leap (Gebo, 1988). In contrast, the trochlea in NMMP 82 is only moderately grooved and its trochlear rims are rounded indicating orderly movements but with some degree of mobility at the talocrural joint. In dorsal view, the trochlea of NMMP 82 appears only slightly wedge shaped, a condition less pronounced than that observed on tali of specialized climbers, which show rather flat and strongly wedged trochlear surfaces allowing much greater mobility (Dagosto, 1983; Gebo, 1988). In NMMP 82, the slightly wider anterior surface of the trochlea relative to its posterior counterpart could have served to limit extreme talocrural dorsiflexion by restricting the forward progress of the tibia, for example, in vertical clinging postures (Gebo, 1988). This action was enhanced by the prominent protuberance developed on the dorsal aspect of the talar neck, which functioned as a tibial stop. The fact that the talar head is wide with its navicular facet extending far posterodorsally onto the talar neck indicates either considerable dorsiflexion of the foot (holding the tibia in place) or strong downward flexion of the tibia (when the foot is grasping a horizontal support). The possibility of enhanced but stable dorsiflexed foot positions suggests that Pondaungia exhibited some proficiency in the use of vertical supports. NMMP 82 lacks a posterior trochlear shelf that acts as a bony stop in extreme plantarflexion at the talocrural joint during a leap (Jouffroy and Gasc, 1974; Gebo, 1988). As in frequently climbing primates, the absence of this feature in NMMP 82 could indicate that leaping was not as important in the locomotor repertoire of Pondaungia (Dagosto, 1983). However, NMMP 82 bears a few talar features indicative of some leaping activity (Gebo, 1988). For instance, the moderately long and nearly straight talar neck, a fairly high talar body, slight trochlear grooving, and a steep medial talotibial facet are the features found in some generalized arboreal quadrupedal primates that are also proficient leapers.
In NMMP 82, the weak lateral expansion of the navicular facet on the talar head reflects limited foot inversion capabilities at the transverse tarsal joint as noted in anthropoids (Fleagle and Meldrum, 1988). Besides, the weak development of the medial talotibial facet, which lacks the medially flaring “cup-like” tibial stop extending onto the medial aspect of the talar neck, also suggests that there was much less stability in sustained habitually inverted foot positions (Gebo et al., 2000). This inverted foot posture probably limited Pondaungia to practicing deliberate locomotion. Pondaungia likely moved by adopting more frequently everted rather than inverted foot postures, preferably along large and flat subhorizontal supports. As such, it seems evident that Pondaungia was probably not a frequent and agile climber. Indeed, NMMP 82 displays a moderately long and nearly straight talar neck, a relatively tall talar body, and shows a moderately grooved trochlea, which is only slightly wedge shaped. These talar conditions contrast markedly with that observed on tali of frequent climbers, which show specialized features promoting much greater mobility for more varied foot orientations associated with climbing activities (i.e., short and medially deflected talar neck, talar body dorsoventrally flat, deep medial malleolar cup extending distally onto the talar neck, weakly grooved to flattened trochlea, and strongly wedged trochlear surface; Dagosto, 1983; Gebo, 1988).
In sum, the NMMP 82 talus displays a suite of talar features that implies intermediate joint mechanics compared with the more extreme morphologies associated with specialized leaping or climbing. These functionally intermediate features indicate a greater emphasis on arboreal quadrupedalism. The foot of Pondaungia seems to be adapted for active quadrupedal movements along broad, subhorizontal branches (or across the ground). Pondaungia was certainly capable of climbing and leaping, although it was not particularly specialized for either of these activities. Similar interpretations derive from the NMMP 39 talus, which shows comparable functional adaptations with perhaps a little more emphasis on leaping. NMMP 39 shows slightly more parallel-sided medial and lateral trochlear walls (e.g., Marivaux et al., 2003). Given the mosaic of postural activities and locomotor behaviors derived from the functional anatomy of the NMMP 82 talus, it remains difficult to identify a compelling living primate as a good analog for Pondaungia in terms of positional behavior, although cebids appear to be a reasonable approximation.
CONCLUSIONS
The morphology of the new primate talus reported here from Thandaung Kyitchaung is close to that of the NMMP 39 talus previously recovered from the same area of the Pondaung Formation (Segyauk Kyitchaung). Both tali exhibit features that resemble talar conditions found in haplorhine primates, notably anthropoids. Neither talus show any morphological link to fossil adapiforms or extant strepsirhines. On the basis of its size and morphology, the NMMP 82 talus is referred to the large-bodied amphipithecid, Pondaungia. The documentation of anthropoid-like postcranial remains from the Pondaung Formation that are appropriate in size to pertain to the large-bodied amphipithecid, Pondaungia is consistent with the anthropoid-like dentition that characterizes all amphipithecids. The apparent conflict between the adapiform-like postcranial elements that occur in NMMP 20 and the anthropoid-like dentition of amphipithecids can now be explained by the presence of two large-bodied primate taxa in the Pondaung Formation: Pondaungia (represented by numerous teeth and jaws from several localities as well as two tali) and a large-bodied adapiform (represented by the NMMP 20 partial skeleton from only one locality but so far undocumented by teeth or jaws). Accordingly, there is no longer any reason to regard the anthropoid-like dentition of amphipithecids as the result of homoplasy. Functionally, the NMMP 82 talus shows intermediate morphological traits indicating a primate that engaged in active arboreal quadrupedalism with some abilities to leap and climb, preferably on large and subhorizontal substrates. However, this primate was apparently not an agile climber in small branches of the upper canopy levels. This specimen provides additional support for the anthropoid status of Pondaungia. However, further specimens are needed for a more complete understanding of the positional behavior and adaptations of this late middle Eocene anthropoid primate from South Asia.
Acknowledgements
The authors thank all the field managers of the international Pondaung Fossil Expedition Team in Myanmar. The authors are particularly indebted to the inhabitants of Mogaung village for their kind hospitality during the field expeditions. Many thanks to Stéphane Dovert, former “Conseiller Culturel” of the French Embassy in Yangon, and Agnes Dovert, for their great interest in our collaboration programme joining Burman, French, Thai, and American scientists. The authors thank Paul Tafforeau (European Radiation Facilities [ESRF], Grenoble) for providing a 3D volume of the NMMP 20 calcaneus (from a cast). Many thanks to Christiane Denys and Jacques Cuisin (Muséum National d'Histoire Naturelle, Paris) and Bill Stanley (Field Museum of Natural History, Chicago) for access to comparative material, Laurence Meslin (Institut des Sciences de l'Évolution de Montpellier, ISE-M) for artworks, and Suzanne Jiquel (ISE-M) for technical assistance. This is publication no 2010- 012 of the “Institut des Sciences de l'Évolution de Montpellier” (ISE-M, UMR-CNRS 5554).




