Chimpanzee Activity and Behavioral Diversity Extends Across 24 Hours in Both Captive and Wild Settings
ABSTRACT
Studying nocturnal behavior is crucial for understanding the full scope of a species' behavioral flexibility so as to inform the conservation of wild populations and the care of captive individuals. However, this aspect of primate behavior is understudied, especially in great apes, which exhibit some of the widest documented behavioral diversity and flexibility. Our investigation is among the first to systematically compare the 24 h activity patterns and behavioral activities of captive chimpanzees (Saint Louis Zoo, USA) with those of wild chimpanzees (three sites across the Nouabalé-Ndoki National Park in the Republic of Congo) and a published data set of the nocturnal behavior of all chimpanzee subspecies. Furthermore, we examined the influence of human activity and changes to the group's composition on the activity patterns and nocturnal behaviors of the zoo-living chimpanzees. Our results reveal that the zoo-living chimpanzees exhibit significantly different activity patterns compared to their wild counterparts, with increased nocturnal activity (particularly in the early morning) and more observations of feeding and social behaviors at night. Additionally, the absence of human visitors and a change in the group's composition were found to influence these activity patterns. These findings underscore the importance of integrating more holistic approaches to captive primate care and wild primate conservation. This study also highlights the immense potential of implementing remote monitoring technology, such as video camera traps, across contexts. Such data that extend across contexts benefit not only the captive and wild great apes but also provide opportunities for caregivers, conservation managers, and students who are involved in these collaborative initiatives.
Summary
-
Captive chimpanzees at the Saint Louis Zoo exhibit significantly different 24 h terrestrial activity patterns and nocturnal activities than their wild counterparts.
-
The 24 h terrestrial activity patterns and nocturnal activities of chimpanzees at the Saint Louis Zoo were significantly impacted by the absence of visitors and changes to their group's composition.
-
Several Pan troglodytes troglodytes populations in the Nouabalé-Ndoki National Park (Republic of Congo) exhibit greater terrestrial nocturnal activity than previously reported.
1 Introduction
Several lines of evidence suggest that animals engage in a variety of activities around the clock, even species described to be most active during daylight hours (i.e., diurnal). Besides resting, nesting, and sleeping, several diurnal primate species have been observed to feed, travel, and even socialize at night (e.g., baboons and macaques: Hammerschmidt et al. 1994; Isbell et al. 2017; Nishikawa and Mochida 2010; Tagg et al. 2018; Vessey 1973; gorillas: Schaller 1963; bonobos: Fruth and Hohmann 1993; and, chimpanzees: Lacroux et al. 2022; Piel 2018; J. D. Pruetz 2018; Tagg et al. 2018; van Lawick-Goodall 1968; Zamma 2014). The study of these behaviors at night is important to understand the full scope of a species' behavioral repertoire (J. Anderson et al. 2019). Additionally, the flexibility of primate behavior in response to environmental and anthropogenic stressors (e.g., habitat loss, human presence, predator density) can be evaluated by assessing changes to their activities throughout the day and night, which helps inform the conservation of species in the wild (Krief et al. 2014; Lacroux et al. 2022) and captive well-being management (Brando, Vitale, and Bacon 2023). However, the degree that nocturnal activity might contribute to population-specific survival, behavioral diversity, and culture is unclear for most primate species. Therefore, it is not certain to what extent nocturnal activities comprise a species' natural behavior and might require conservation action (e.g., Carvalho et al. 2022). This is particularly true for chimpanzees, who exhibit some of the widest documented behavioral diversity and cultural complexity in the animal kingdom across a vast array of ecological contexts (Kalan et al. 2020). Not only is the conservation of wild chimpanzees and their behavioral diversity critical (Carvalho et al. 2022; Kühl et al. 2019), but so is the continued pursuit of information to provide the highest standard of care for individuals who live in captive environments (Mellor 2016; J. Pruetz and McGrew 2001; Ross 2020). With only limited published data on wild chimpanzee nocturnal activities, however, evaluating the effectiveness of captive environments in encouraging the full spectrum of chimpanzee behaviors is challenging (Angley, Vale, and Cronin 2024; Brando, Vitale, and Bacon 2023; J. Pruetz and McGrew 2001).
While most captive primate management philosophies promote the mirroring of captive conditions to those of wild populations (J. Pruetz and McGrew 2001; Ross 2020; Wolfensohn et al. 2018), overnight activities likely vary between wild and captive chimpanzees due to differences in key environmental factors such as the relative absence of food scarcity and predation pressure in captive settings (Tagg et al. 2018). Of even greater concern is the fact that very little is known about what captive chimpanzees do at night, aside from resting and retiring behavior (e.g., Earl, Hopper, and Ross 2020; Morimura et al. 2012; Videan 2006). Accounting for more than 33% of their total 24 h days, however, the overnight activity of chimpanzees likely impacts their well-being. For example, longer periods of nocturnal aggression among sanctuary-living chimpanzees, as measured via remote audio recordings, correlate with increased observations of inactivity, abnormal, self-directed, and affiliative interactions throughout the following day (Ayuso et al. 2023). Additionally, nocturnal activity has been reported to increase during and directly after stressful social events (e.g., integration into a new group: Morimura et al. 2012). We advocate that more holistic comparisons of chimpanzee activities in wild and captive settings require 24 h monitoring with methods that allow for quantification of variation across individual apes. Such enhanced monitoring is now possible through remote video technology and has the potential to provide a more complete understanding of chimpanzee behavior across the 24 h day, which is critical information for designing optimal environments that allow zoo- and sanctuary-living individuals to thrive (Brando and Buchanan-Smith 2018; Brando, Vitale, and Bacon 2023; Mellor 2016).
This field of research has been limited by the abilities of human observers to longitudinally record the nighttime behavior of primates as they would using in-person data collection techniques during daylight hours due to safety concerns and logistical constraints (J. D. Pruetz 2018; Zamma 2014). However, in recent years, passive infrared video camera traps have provided a means to achieve more comprehensive monitoring even with animals that are not habituated to human presence (Lacroux et al. 2022; Tagg et al. 2018). For example, in a widespread video camera trap evaluation of wild chimpanzees' nocturnal activity, observations of active chimpanzees at night appeared to be infrequent but relatively equally abundant across study sites and subspecies (approx. 1.8% of average activity, observed at 18 out of 22 sites: Tagg et al. 2018). Using these methods to study wild chimpanzees, there is evidence that lower levels of human activity, higher average daily temperature, and greater forest cover contribute to overall patterns of chimpanzee activity at night, whereas the abundance of predators and other large mammals (i.e., niche competitors), rainfall, and moon illumination had little effect (Tagg et al. 2018). During the day, however, individual chimpanzees do not display the same repertoires of behavior, and therefore, it is unlikely that all chimpanzees exhibit the same patterns of activity and repertoires of behavior at night. Without the ability to record the behavior of identifiable individuals across time, though, little can be said about the possible interindividual variability in chimpanzees' activity patterns and nocturnal behavior that is critical to protecting their behavioral diversity and devising effective care plans for captive individuals.
In this investigation, we used passive infrared video camera traps to systematically compare the terrestrial nocturnal activities of chimpanzees across captive and wild settings. To do so, we used data from video camera traps at the Saint Louis Zoo (Missouri, USA) and in the Nouabalé-Ndoki National Park (Republic of Congo; Morgan et al. 2023) as well as a previously published data set of wild chimpanzee behavior across multiple study sites (Tagg et al. 2018). We predicted that zoo-living chimpanzees are more active at night and display a wider diversity of activities compared to their wild counterparts. Furthermore, we predicted consistent, interindividual differences in the nighttime activities of the zoo-living chimpanzees. Finally, we predicted that the activity patterns of the zoo-living chimpanzees would vary in association with human activities and changes to the composition of their social group.
2 Methods
2.1 Study Site and Subjects
The chimpanzee group at the Saint Louis Zoo (Missouri, USA) was composed of nine adult individuals (N = 6 females, 3 males) during our data collection period from September 2019 to September 2022. Additionally, Utamu, an adult female, gave birth to one infant female in October 2020. The Saint Louis Zoo chimpanzee group has consistent access to a one-quarter-acre outdoor habitat (on public display) as well as multiple enclosure spaces (off public display) and one indoor habitat (on public display) throughout the day and night. All chimpanzees are served multiple meals of a diverse array of fruits, vegetables, greens, browse, forage items, nuts, seeds, and other prepared foods, as well as provided with multiple daytime opportunities for environmental (e.g., nesting material) and food puzzle enrichment. While food and enrichment items are dispersed during the day, many of these items remain available throughout the night (e.g., uneaten foods and enrichment items that have not been depleted of their food rewards).
2.2 Data Collection
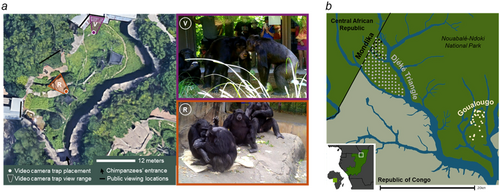
We deployed high-definition, motion-activated video camera traps at two locations of frequent use and higher density of observed social behavior within the outdoor chimpanzee habitat at the Saint Louis Zoo beginning in 2019. Bushnell Essentials E3 video camera traps were programmed to record for 60 s in color or 15 s in black-and-white after activation, with a minimum of 1 s between consecutive activations and in the default motion-detection sensitivity mode. The cameras make adaptive decisions about when to record in color versus black-and-white based on lighting conditions sensed at the time of activation. The video camera traps were retrofitted with plexiglass protective coverings for the infrared light array and camera lens. All removable mesh hardware pieces and plastic coverings were permanently glued into place. Both cameras were mounted in Bushnell-manufactured metal protective housing units and locked into place for use (Figure 1). The camera batteries and memory cards were exchanged approximately once per month. At no point during data collection were any video camera traps damaged by the chimpanzees in any way that would affect the camera's functionality. All footage was stored on external hard drives, then linked, screened, and scored in INTERACT version 18.7 (Mangold 2017).

To systematically and exhaustively compare the nocturnal activity patterns by wild and zoo-living chimpanzees, we compared video camera trap data from the Saint Louis Zoo with data from wild chimpanzees sampled for various durations with slightly different camera trap placements schemes from across the Nouabalé-Ndoki National Park (Republic of Congo): at environmental features (termite nests at the Goualougo study site from 2019 to 2020, Morgan et al. 2023), at environmental features and a grid (termite nests and small grid at the Mondika study site from 2017 to 2020, Morgan et al. 2023), as well as at locations that were randomly determined based on a systematic grid that covered the entire study area with no environmental features dictating camera placements (across the Djéké Triangle in 2021). These data were collected by high-definition, motion-activated video camera traps with identical camera configurations and settings. All camera traps were placed approximately 1 m off the ground to produce camera trap footage of the chimpanzees' terrestrial activities similar to that produced by cameras at the Saint Louis Zoo. A complete overview of the data sets and camera traps used in this investigation is provided in Table 1, and a map of all camera trap placements is provided in Figure 2.
| Population, study site | CT placement | Number of CTs | Duration (months) | Chimpanzee clips | Source |
|---|---|---|---|---|---|
| Zoo-living chimpanzees | |||||
| Saint Louis Zoo, USA | Areas of frequent use | N = 2 | 37 | N = 50,205 | [1] |
| Wild chimpanzees | |||||
| Goualougo Study Site, Republic of Congo | Termite nests | N = 29 | 24 | N = 1015 | [2] |
| Mondika Study Site, Republic of Congo | 1 km grid + termite nests | N = 19 | 36 | N = 1088 | [2] |
| Djéké Triangle, Republic of Congo | 1 km grid | N = 100 | 4 | N = 117 | [1] |
| PanAf (N = 39 sites) | 1 km grid | N = 5−41 | 7−22 | N = 10,155 | [3] |
- Note: CT is an abbreviation for video camera trap. The data collected at the Mondika study site (2017−2020) and the Djéké Triangle (2021) represent independent data sets, as these cameras were not deployed during the same time period. References: [1] This study; [2] Morgan et al. 2023; [3] Tagg et al. 2018.
All research reported in this manuscript complied with the protocols approved by the Washington University in St. Louis Institutional Animal Care and Use Committee, the Saint Louis Zoo Research Review Committee, the Nouabalé-Ndoki Foundation, the Wildlife Conservation Society's Congo Program, and the legal requirements of the Republic of Congo. This research also adhered to the American Society of Primatologist's Principles for the Ethical Treatment of Non-Human Primates, the International Primatological Society's Code of Best Practices for Field Primatology, as well as the Ethical Guidelines of the Association for the Study of Animal Behaviour and the Animal Behaviour Society.
2.3 Data Analysis
To test for differences in activity patterns across the zoo-living and wild chimpanzees, we used the timestamp for each camera trap video to determine the activity density per hour for chimpanzees across the Saint Louis Zoo, Goualougo, Mondika, and Djéké Triangle data sets (Cappelle et al. 2021; Morgan et al. 2023). We converted the recorded time of each clip to radian time-of-day data and fit the data to a Von Mises kernel density distribution using the fitact() function in the “activity” package in R with a bandwidth multiplier of 1.5 (Rowcliffe et al. 2014). This function fits a comparable smoothed distribution with confidence limits to the circular radian time-of-day data to estimate the proportion of the time during a full 24 h camera-day where an animal was active. Therefore, these analyses are well-suited for comparisons across populations of different sizes sampled with slightly different video camera trap placement schemes. The bandwidth adjustment we chose reflects the recommendation of previous authors who were also interested in producing representative, systematic comparisons (Rowcliffe et al. 2014). We excluded data for camera trap videos that were recorded within 5 min of the previous video to ensure independence between detection events for analyses of 24 h activity patterns (Morgan et al. 2023). We replicated these analyses with data from each adult individual at the Saint Louis Zoo. We then used the compareAct() function also in the “activity” package to test for differences among population-specific patterns of activity across the zoo-living and wild populations as well as across the zoo-living individuals. This function uses a Wald null-hypothesis significance test to determine whether the difference between circular activity estimates is different from zero on a Chi-squared distribution with a degree of freedom of one, and which is appropriate for comparing both overall activity levels and activity levels at specific times of day (Rowcliffe et al. 2014).
To compare patterns of the observed nocturnal behavior between zoo-living and wild chimpanzees, we used the same method of behavioral data collection as similar investigations in wild chimpanzee nocturnal activity (Tagg et al. 2018). We classified the Saint Louis Zoo chimpanzees' activity into four categories: movement, social, feeding, and “on location” (climbing, sitting, standing: Tagg et al. 2018). Thereafter, we coded further details about the behavior of each individual, including object manipulation (touching, inspecting, or otherwise manipulating an attached or detached object in a non-feeding context; e.g., nest construction), inactive (sitting, resting, sleeping), self-grooming (manipulating one's own hair or skin), social grooming (manipulating the hair or skin of at least one other conspecific), social play (including play push, bite, slap, chase, and grab with play face and/or breathy pant vocalization), other affiliation (e.g., greetings, genital inspection, copulation), other social behavior (e.g., pant-hooting), and not visible behavior (i.e., when individual chimpanzees are identifiable but only visible for a few seconds and their behavior is unknown). Of these additional behaviors, when comparing our data with those of published data on wild chimpanzees (Tagg et al. 2018), we grouped not visible behavior with “movement”; social grooming, social play, other affiliation, and other social behavior into “social”; object manipulation with “feeding”; and, self-grooming and inactive into “on location.” We considered camera trap videos to be recorded at night if the cameras were activated within 30 min of sunset through within 30 min of sunrise (Tagg et al. 2018). Sunrise and sunset times for each date were compiled using the getSunlightTimes() function in the “suncalc” package (Thieurmel 2022). We used a Chi-square test of independence and Fisher's exact test to evaluate any differences in the proportion of nocturnal behaviors exhibited by chimpanzees at the Saint Louis Zoo and wild chimpanzees (as reported by Tagg et al. 2018).
During a period of data collection at the Saint Louis Zoo, the zoo was closed to the public due to the COVID-19 pandemic. The complete absence of public visitors during this time provides us with an opportunity to evaluate the potential influence of human activity on the chimpanzees' activity patterns. We used the same compareAct() function in the “activity” package to test for differences in their activity patterns between March 17, 2020 and June 13, 2020, when the zoo was closed and the same time 1 year later after the zoo had reopened (March 17, 2021 to June 13, 2021; similar to methods employed by Edes et al. 2022). Additionally, during the period of data collection at the Saint Louis Zoo, the chimpanzee group composition changed due to the loss of one adult female, Mlinzi. The change in group composition provides us with an additional natural, opportunistic opportunity to evaluate the potential influence of social uncertainty (i.e., perturbation to group composition) on the chimpanzees' activity patterns. We used the same compareAct() function in the “activity” package to test for differences in their activity patterns after the change to their group composition (July 7, 2022) to the end of data collection for this investigation (September 16, 2022) with their activity during this same period 1 year prior (July 7, 2021 to September 16, 2021). We constructed both comparative tests with matched data from the same time during the previous or subsequent year to ensure similarity in weather (e.g., temperature, rainfall, moonlight). We then used a Chi-square test of independence and Fisher's exact test to evaluate any differences in the proportion of nocturnal behaviors exhibited by chimpanzees at the Saint Louis Zoo in both comparisons. All analyses were conducted in R version 4.3.3.
3 Results
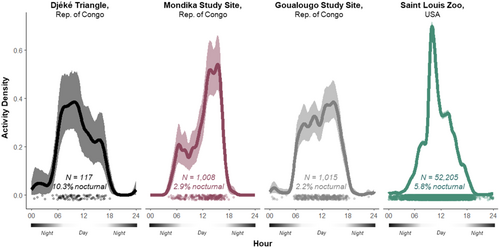
From more than 3 years of video camera trap data recorded in the outdoor habitat of the chimpanzees at the Saint Louis Zoo, we observed the captive chimpanzees in 2680 videos that were recorded at night and 49,525 that were recorded during the day (N = 52,205, 5.8% nocturnal). Example observations of the terrestrial nocturnal activities of chimpanzees at the Saint Louis Zoo are presented in Figure 3. From 2 years of data recorded at termite nests at the Goualougo Triangle study site, we observed wild chimpanzees in 22 videos that were recorded at night and 993 that were recorded during the day (N = 1015, 2.2% nocturnal). From 3 years of data recorded at termite nests and across a sampling grid at the Mondika study site, we observed wild chimpanzees in 32 videos that were recorded at night and 1056 that were recorded during the day (N = 1088, 2.9% nocturnal). From 4 months of data recorded at locations that were randomly determined based on a systematic grid across the Djéké Triangle, we observed wild chimpanzees in 12 videos that were recorded at night and 105 that were recorded during the day (N = 117, 10.3% nocturnal). Example observations of the terrestrial nocturnal activities of chimpanzees in the wild observed in this investigation are presented in Figure 4.


3.1 Activity Budgets Across Captive and Wild Settings
The results of our test of differences in the 24 h activity patterns across zoo-living and wild populations indicate that the activity patterns of Saint Louis Zoo chimpanzees were significantly different from all wild chimpanzee populations included in this investigation: Saint Louis Zoo–Goualougo = −0.07, SE = 0.02, W = 8.38, p < 0.001; Saint Louis Zoo–Mondika = −0.19, SE = 0.29, W = 41.23, p < 0.001; Saint Louis Zoo–Djéké Triangle = −0.19, SE = 0.04, W = 17.80, p < 0.001 (Figure 5). Noticeably, the chimpanzees at the Saint Louis Zoo were observed much more frequently to be active during the early hours of the morning compared to their wild counterparts, with a much proportionally stronger peak in their activity before 1200.
3.2 Nocturnal Behaviors Across Captive and Wild Settings
The distribution of the Saint Louis Zoo chimpanzees' nocturnal behaviors was significantly different from those previously reported by wild chimpanzees (Tagg et al. 2018): χ2 = 27.07, DF = 3, p < 0.001; Fisher's exact test, p < 0.001. The same result is true for comparisons across zoo-living and wild male chimpanzees (χ2 = 12.87, DF = 3, p = 0.005; Fisher's exact test, p < 0.01) and female chimpanzees (χ2 = 21.77, DF = 3, p < 0.001; Fisher's exact test, p < 0.01). Figure 6 illustrates the differences in the observed nocturnal activity of wild and zoo-living chimpanzees, and Table 2 reports the exact number of observations for each nocturnal activity. Noticeably, the Saint Louis Zoo chimpanzees were more often feeding and socializing at night than their wild counterparts.
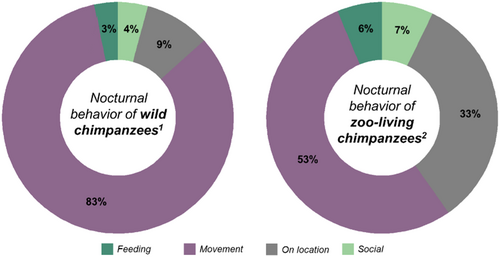
| Behavior | Wild chimpanzees1 | Zoo-living chimpanzees2 | ||
|---|---|---|---|---|
| Adult males | Adult females | Adult males | Adult females | |
| Feeding | 3 | 0 | 62 | 144 |
| Movement | 49 | 42 | 585 | 1459 |
| On location | 14 | 5 | 347 | 854 |
| Social | 4 | 0 | 201 | 165 |
| Total | 70 | 47 | 1195 | 2622 |
- Note: [1] Includes only data from Tagg et al. 2018; [2] This study at the Saint Louis Zoo.
3.3 Interindividual Variation in Zoo-Living Chimpanzee Activity Patterns
Results of the pair-wise Wald test of interindividual variation of nocturnal activity patterns indicate that the activity patterns of some adult individuals at the Saint Louis Zoo were significantly different from conspecifics (e.g., Jimiyu and Bakhari compared to all others). Table S1 provides the entire results of these pair-wise Wald tests of the compareAct() analyses. Key findings include that Jimiyu was observed less in the early morning hours than almost all of his conspecifics (i.e., in the midnight to 0300 h; difference = −0.0.5 ± 0.02, SE = 0.02 ± 0.002, W = 11.21 ± 6.48) and Bakhari was observed more often at night overall than a few of her conspecifics (i.e., around 1800 and later; Rosebud, Tammy, Utamu, and Beauty, difference = 0.003 ± 0.05, SE = 0.02 ± 0.001, W = 6.54 ± 2.49). Of note, we did not detect any patterns of differences in these pair-wise comparisons of interindividual differences across individuals who were older or younger nor male or female (see Table S1). Figure 7 illustrates the differences in observed nocturnal activity patterns of the adult chimpanzees at the Saint Louis Zoo along with their behavioral activities as collected with our expanded ethogram. Table 3 reports the exact number of observations for each nocturnal activity for all individuals.
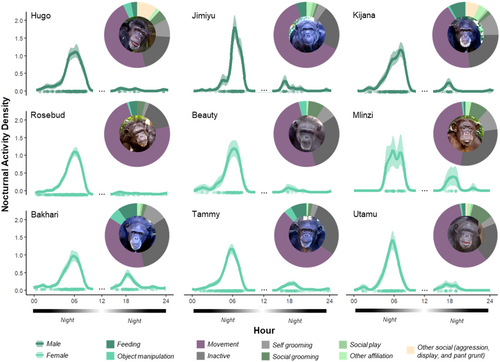
| Behavior | Adult males | Adult females | Immature female | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hugo | Jimiyu | Kijana | Rosebud | Beauty | Mlinzi | Bakhari | Tammy | Utamu | Raven | |
| Feedinga,1 | 11 | 16 | 35 | 31 | 0 | 1 | 61 | 44 | 7 | 10 |
| Object manipulationb,1 | 16 | 1 | 4 | 9 | 7 | 1 | 33 | 20 | 3 | 14 |
| Movement2 | 199 | 187 | 199 | 604 | 93 | 51 | 221 | 291 | 199 | 64 |
| Inactive3 | 80 | 67 | 109 | 123 | 55 | 33 | 180 | 160 | 68 | 46 |
| Self grooming3 | 35 | 13 | 49 | 19 | 12 | 12 | 67 | 18 | 12 | 5 |
| Social grooming4 | 21 | 3 | 11 | 35 | 15 | 10 | 10 | 11 | 38 | 1 |
| Social play4 | 0 | 5 | 3 | 0 | 0 | 0 | 3 | 0 | 2 | 2 |
| Other affiliationc,4 | 7 | 9 | 19 | 4 | 1 | 3 | 10 | 6 | 7 | 3 |
| Other social4 | 40 | 0 | 35 | 5 | 0 | 0 | 2 | 0 | 2 | 0 |
| Not visible behavior2 | 10 | 5 | 6 | 5 | 1 | 4 | 7 | 6 | 0 | 1 |
| Total | 419 | 306 | 470 | 835 | 184 | 115 | 594 | 556 | 338 | 146 |
- Note: We did not observe any individuals sleeping outside nor resting in the same location for any longer than a few minutes. To compare these data with previously published data on wild chimpanzees (Tagg et al. 2018), behaviors were grouped into 1feeding, 2movement, 3on location, and 4social behaviors.
- a Feeding includes three observations of coprophagy.
- b Object manipulation includes 33 observations of individuals manipulating nesting materials (e.g., blanket, burlap, wood wool) and 11 observations of individuals manipulating enrichment objects or toys.
- c Other affiliations include five observations of copulation by five unique dyads (including two males and three females).
3.4 The Effect of Visitors on Zoo-Living Chimpanzee Activity Patterns and Nocturnal Behavior
The results of our test for differences in the 24 h activity patterns of the zoo-living chimpanzees with and without the presence of human visitors indicate that the chimpanzees at the Saint Louis Zoo exhibited significantly different patterns of activity across these two conditions: COVID-19 closure in 2020—matched sample from 2021 = 0.02, SE = 0.01, W = 5.89, p = 0.015. Notably, the chimpanzees appear to have been more active during the early morning hours during the period when the zoo was closed and no public guests were present (Figure 8a). Further, the distribution of the Saint Louis Zoo chimpanzees' nocturnal behavior during the COVID-19 closure was slightly different from those observed in the matched sample 1 year later (χ2 = 13.21, DF = 6, p = 0.040; Fisher's exact test, p = 0.059) with a higher diversity of behaviors and less inactive behaviors observed in the absence of human visitors.
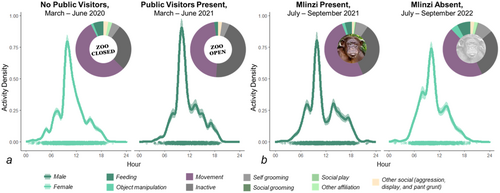
3.5 The Effect of Social Uncertainty on Zoo-Living Chimpanzee Activity Patterns and Nocturnal Behavior
Similar tests for differences in the activities of the Saint Louis Zoo chimpanzees 1 year before a change in the group's composition (i.e., the loss of Mlinzi) and directly after indicated significantly different distributions of nocturnal activities (χ2 = 27.77, DF = 9, p = 0.001; Fisher's exact test, p < 0.001) but no significant difference in their 24 h activity patterns (Mlinzi present–Mlinzi absent: 0.02, SE = 0.01, W = 1.89, p = 0.17). Notably, after the change to the group's composition, the chimpanzees exhibited an increased proportion of feeding and social behavior at night (Figure 8b).
4 Discussion
In this study, we aimed to compare the 24 h activity patterns and nocturnal behavior of captive and wild chimpanzees using video camera traps. Our results indicate that the terrestrial activity patterns of chimpanzees at the Saint Louis Zoo (5.8%, N = 2680/52,205 nocturnal observations) differ significantly from those of wild populations, with the zoo-living chimpanzees exhibiting a higher frequency of nocturnal activity (particularly in the early morning hours) although the highest proportion of nocturnal activity was observed in the Djéké Triangle. Our results contribute to a greater understanding of the population-specific behavioral variation of Pan troglodytes troglodytes as the data from the Goualougo (2.2%, N = 22/1015 nocturnal observations) and Mondika (2.9%, N = 32/1088 nocturnal observations) study sites as well as across the Djéké Triangle (10.3% N = 12/117 nocturnal observation) indicate much greater nocturnal activity than previously reported for this subspecies (Tagg et al. 2018, P. t. troglodytes: mean = 0.41%, SD = 0.50, N = 7/958 nocturnal observations). Furthermore, we observed that zoo chimpanzees engage more often in feeding and social behavior at night than their wild counterparts. In addition, there are more observations of chimpanzees awake but inactive among the zoo compared to wild chimpanzees. The prevalence of terrestrial nocturnal behaviors we observed in this study is significant given that they represent choices by wild and captive chimpanzees to leave their night nest and engage in other activities for various reasons. At the Saint Louis Zoo, we also observed significant interindividual variation in the chimpanzees' nocturnal activity patterns. Interestingly, the presence of human visitors influenced the zoo-living chimpanzees' activity patterns, as they were slightly more active at night when the zoo was closed to the public due to the COVID-19 pandemic. Moreover, changes in the social group composition also resulted in altered nocturnal behaviors, particularly more social behaviors. These findings underscore the complex interactions between environmental, social, and anthropogenic factors on chimpanzee behavior, with implications for their conservation and well-being management in captivity.
Adopting a 24 h approach to individual well-being is important for providing zoo- and sanctuary-living individuals the opportunity to thrive (Brando and Buchanan-Smith 2018; Brando, Vitale, and Bacon 2023; Mellor 2016). Toward this aim, we describe the first comprehensive report of the 24 h activity patterns and nocturnal behavioral repertoires of captive chimpanzees. One important aspect of captive chimpanzee well-being is to ensure that individuals can exhibit behaviors that are similar to their wild counterparts (Inoue and Shimada 2020; J. Pruetz and McGrew 2001). Therefore, we also conducted systematic comparisons of the activity budgets and nocturnal behavior of wild chimpanzees. We detected that the Saint Louis Zoo chimpanzees were much more active at night, particularly in the early morning hours, compared to individuals in wild populations. However, we also observed the captive chimpanzees to travel less than wild individuals at night, while engaging more frequently in feeding, social behaviors, and “on location” behaviors (sensu, Tagg et al. 2018). We did not observe any captive individuals sleeping at all outside nor resting outside in the same location for more than a few minutes. Taken together, this information can be used to inform captive care management plans should overall activity patterns or species-typical nocturnal behavior be identified as priorities for well-being. For example, in considering overnight cognitive enrichment that might encourage naturalistic behaviors similar to their wild counterparts when the chimpanzees are awake at night (e.g., Yamanashi and Hayashi 2011; Yamanashi et al. 2016) and dispersing nesting material in the outdoor habitat to encourage nest construction and sleeping outdoors (e.g., blankets, burlap, wood wool: N. Anderson, Amarasekaran, and Riba 2021; Fultz et al. 2013; Morimura et al. 2012; Videan 2006). Nonetheless, while the well-being implications of our results remain the topic for future investigations, it appears that access to their outdoor habitat is an important part of these chimpanzees' captive care plan that facilitated species-typical wild-type behaviors. We predict future investigations in other species will find similar effects of overnight access to and use of outdoor spaces, which has already been documented to promote the positive welfare of zoo-living animals (e.g., Powell and Vitale 2016).
Providing captive animals with choice over their access to and use of their entire environment has considerable implications for their expression of agency and well-being (Duncan, D'Egidio Kotze, and Pillay 2022; Ross et al. 2011; Rust, Clegg, and Fernandez 2024; Scott and LaDue 2019). The size and characteristics of enclosure spaces impact chimpanzee well-being and social behavior (Duncan, D'Egidio Kotze, and Pillay 2022; Herrelko, Buchanan-Smith, and Vick 2015; Neal Webb, Hau, and Schapiro 2018; Ross et al. 2011). Particularly during periods of social uncertainty, an increase in the density of captive individuals in inside-only enclosure spaces can lead to deleterious aggression and stereotypic behavior (Duncan et al. 2013; Duncan, D'Egidio Kotze, and Pillay 2022) and an increase in perceived available space can aid in reducing stress-related responses during the integration of new chimpanzees to a group (Herrelko, Buchanan-Smith, and Vick 2015). In several hundred video camera trap clips, we observed individuals at the Saint Louis Zoo pant-hooting (with pilo-erect hair). In most of these videos, vocalizations that are typical of social conflict could be heard coming from other individuals in the distance. These observations most often occurred at times when the cameras had not been motion-activated for several hours, indicating that these social events did not start outdoors. The frequency of these types of observations indicates to us that the chimpanzees likely use their available outdoor space at night to mitigate aggression or social tension, similar to results and suggestions posed by other authors (Ayuso et al. 2023; Duncan et al. 2013). The significant increase in observed nocturnal social behavior during a period of social uncertainty after Mlinzi's passing supports this interpretation. Of considerable interest would be future investigations into the extent to which wild chimpanzees engage in aggressive behavior or behaviors indicative of social uncertainty overnight. However, the rarity of these types of interactions by wild chimpanzees prohibits this topic from being considered systematically in this study.
The results of our investigation suggest that providing chimpanzees with overnight access to their outdoor habitats is important to their well-being. These benefits likely accompany the positive effects that are associated with providing greater agency and choice to captive individuals so that they can make self-determined decisions about where and with whom to spend their time (Angley, Vale, and Cronin 2024; Herrelko, Buchanan-Smith, and Vick 2015; Rust, Clegg, and Fernandez 2024). Of note, we observed almost no stereotypic behavior exhibited by the Saint Louis Zoo chimpanzees at night. Possible exceptions to this are a total of three instances of coprophagy by two adult females, though it should be noted that coprophagy rarely informs a meaningful assessment of captive apes' well-being (Hopper, Freeman, and Ross 2016). Additionally, we observed a total of five copulations at night by five unique dyads (composed of two males and three females). These observations are in stark contrast to the rarity of these observations during the day, either in person or via the video camera trap footage. Given that concealed copulations have been observed in the wild (e.g., Nishida 1980; Townsend, Deschner, and Zuberbühler 2008) and chimpanzees are capable of deception (e.g., Karg et al. 2015), our observations of copulations at night further illuminate the flexibility of chimpanzee behavior. Based on our findings, we support the suggestion that captive chimpanzees should be afforded as much agency and choice as possible to behave flexibly in response to local social and ecological pressures. Ensuring overnight access to the entirety of available space and monitoring of overnight behavior are important aspects of this type of agency and choice. We predict future investigations interested in flexible behavioral responses and 24 h behavioral diversity will find that greater agency along these dimensions provides long-term benefits to individual well-being and have important evolutionary implications. However, such effects will continue to be overlooked in the absence of systematic assessments of nocturnal activities.
We joined several other authors who used zoo closures during the COVID-19 pandemic to take a closer look at how human visitors might influence these animals (e.g., Edes et al. 2022; Frost et al. 2022; Masman et al. 2022; Williams et al. 2021). In the absence of public guests at the Saint Louis Zoo, the chimpanzees showed a more gradual decline in activity directly after 1200, were more active in the early morning, and displayed a greater diversity of nocturnal behaviors. While there is no suggestion from these data that the presence or absence of public visitors drastically affected the activity patterns and nocturnal behavior of these individuals so as to indicate direct impacts on their well-being, additional detailed investigations of their behavior in relation to public visitation schedules are warranted. Additionally, we recognize that the absence of human visitors alone might not entirely explain the effects we describe here. For example, several other variables could have influenced the chimpanzees' activity patterns, such as modifications to the work schedule of caregivers, other modified zoo operations, or enhanced health and safety protocols. While a chorus of investigations have described similar differences in captive animal behavior in the absence of humans (e.g., Edes and Hall 2023; Hosey, Ward, and Melfi 2023; Williams et al. 2023), the mechanisms that drive such changes and the implications of these differences for understanding the behavioral ecology, flexibility, and cognition of different species remain to be determined. We encourage future research that aims to identify the relative contribution of specific variables to producing a population-level change in zoo and sanctuary-living primate behavior.
We urge for continued research that focuses on within-species comparisons of captive and wild populations as well as cross-species comparisons to provide a more holistic understanding of how environmental features, species' natural propensities, and individual preferences interact to produce 24 h activity patterns and behavioral repertoires. For wild primates, it is likely that emerging technologies will make nocturnal observations of their behavior and nesting strategies more feasible (e.g., night vision, infrared, and thermal drones). However, densely forested environments will likely continue to pose considerable challenges for investigations of this topic. We also hope that the growing collection of nocturnal observations of wild chimpanzees feeding, traveling, and even socializing prompts investigations of this topic across study sites (Lacroux et al. 2022; Piel 2018; J. D. Pruetz 2018; Tagg et al. 2018; van Lawick-Goodall 1968; Zamma 2014). For captive primates, a multidimensional comparative approach could prove fruitful in illuminating new insights into habitat design and standards of care (e.g., Earl, Hopper, and Ross 2020). While video camera trap methods have become common in field studies of wild animals (e.g., Caravaggi et al. 2020), their use does not seem to be as widespread in captive environments. However, video camera traps can likely aid in facilitating systematic comparisons across species, populations, and environments. Our investigation demonstrates the impact of using passive infrared and motion-sensor video camera traps in captive environments to inform management and well-being. This technology is not only cost-effective and efficient but also produces videos that are amenable to artificial intelligence applications and machine learning algorithms that have the potential to further improve data yields (e.g., individual recognition: Guo et al. 2020; McCarthy et al. 2018; Schofield et al. 2019; behavioral classification: Marks et al. 2022; Wiltshire et al. 2023).
While our two video camera traps in the outdoor habitat at the Saint Louis Zoo were essential for providing systematic comparisons with wild apes, we acknowledge that they likely provide a somewhat limited glimpse of the full depth of species-typical nocturnal activity. For example, minor variations in camera trap placement schemes (i.e., areas of frequent use or grid) and different habitat sizes (i.e., 100 km2 in the Djéké Triangle, 0.001 km2 at the Saint Louis Zoo) might indicate that our comparisons across populations should be interpreted with some degree of caution. Some of our reported differences across settings could be an artifact of terrestrial video camera trapping methods. For example, the location and density of risks to wild chimpanzees might reduce the likelihood that these cameras would record them socializing on the ground at night, even if such socializing is occurring in an arboreal context. Similarly, the view that these data give us insight into the full breadth of interindividual differences might be disproportional if the behavior of some individuals correlates with any unique patterns of nocturnal habitat use. For future investigations, we advocate for broader coverage of behavioral surveys with camera traps or other emerging technology across all habitat types, elevations, and nocturnal environmental variables (e.g., lunar illumination). It may also be interesting to examine if the chimpanzees' perceived risk of their terrestrial nocturnal behaviors (e.g., vigilance behavior, social conflicts, terrestrial nesting) influence their interindividual differences in nocturnal behavioral repertoires.
We anticipate the incorporation of population-specific nocturnal activity patterns and behavioral repertoires into conservation management planning, particularly as it relates to longitudinal monitoring and the preservation of species-typical behavioral diversity (i.e., culture: Carvalho et al. 2022). Finally, we are looking forward to the proliferation of next-generation video camera trap methods and streamlined analysis pipelines that will expedite the production of results from vast arrays of cameras across wild and captive settings. Such advancements will revolutionize our ability to conduct individual well-being assessments for captive individuals and conserve the behavioral diversity of wild populations in near real-time, which is essential for improving the outlooks of many populations and species.
Acknowledgments
We are grateful for the opportunity to conduct this research at the Saint Louis Zoo. Our sincere thanks to D. Strunk, T. Sehnert, J. Kehlenbrink, E. Riepl, B. Radman, V. Alm, M. McElya, K. Emerson, and the entire Jungle of the Apes caregiving team for their assistance in camera trap installation and their continued support of this work. We also thank R. Williams, R. Derby, O. Blumenshine, S. Good, L. Greenberg, C. Keuchel, M. Matlock, and L. Shpiz for their contributions to the research at the Saint Louis Zoo. Further, we are grateful for the support, review, and feedback provided by D. Powell and the Saint Louis Zoo Research Review Committee on the protocols used in this investigation. Additionally, we deeply appreciate the opportunity to work in the Goualougo and Djéké Triangles within the Nouabalé-Ndoki National Park. This research would not have been possible without the continued support of the Ministère de l'Economie Forestière du gouvernement de la République du Congo and the Agence Congolaise de la Faune et des Aires Protégées (ACFAP). The Wildlife Conservation Society's Congo Program and the Nouabalé-Ndoki Foundation are integral partners in this continuing research. Special thanks are due to M. Gately, E. Stokes, V. Estienne, T. Breuer, P. Ngouembe, D. Dos Santos, E. Arnhem, M. Ngangoue, M. Mviri, and B. Evans. We would also like to recognize the tireless dedication of J. R. Onononga, S. Ndolo Ebika, S. Kialiema, J. Wawa, D. Koni, M. Meguessa, A. Zambarda, I. Singono, J. Ortega, S. Brogan, and the entire Goualougo and Mondika research teams. Grateful acknowledgment of funding is due to the Arcus Foundation, the Conservation, Food, and Health Foundation, the Indianapolis Zoo, the Cincinnati Zoo and Botanical Garden, the Saint Louis Zoo, and the Animal Behavior Society. Special thanks are due to our review editor, K. Gartland, for the invitation to participate in this special issue. Our thanks to K. Gartland, C. Stephens, and the two anonymous reviewers for their thoughtful reviews of this manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available upon request.




