Hand Preferences in Olive Baboons (Papio anubis) During Cognitive Performance on Match-to-Sample Tasks and Natural Behaviors
ABSTRACT
An individual shows handedness when they consistently prefer one hand over the other for tasks that can be performed with either hand. Humans have a population-level right-hand preference, and past research shows that a variety of nonhuman primate species also show hand preferences. More complex manual tasks elicit stronger hand preferences than less complex manual tasks, but not much is known about hand preferences during a cognitive task in nonhuman primates. The current study investigated hand preferences in olive baboons (Papio anubis). Seven baboons participated in a match-to-sample task on a touchscreen computer. We recorded each baboon's hand use as they touched the start box, sample stimulus, choice stimulus, and which hand they used to retrieve the food reward. All 10 baboons in the troop were also observed in their outdoor zoo habitat, where they were carrying out natural behaviors. In the current study, the touchscreen task was cognitively demanding, without being manually complex, as the baboon simply touched the screen. The direction and strength of hand preference were calculated using z-scores and handedness index (HI) scores for each individual baboon. When completing the cognitive task, five baboons were left-handed, and two baboons were right-handed. Five of the baboons had strong preferences (three left-handed and two right-handed) and two had weak preferences. When engaging in natural behaviors, eight baboons were left-handed, one baboon was right-handed, and one baboon was ambiguously handed. Two of the baboons had strong preferences (one right-handed and one left-handed), and eight had weak preferences. Four of the seven baboons had consistent hand preferences when completing the cognitive task and when engaging in natural behaviors in their habitat. These results show that similar to manually complex tasks, a complex cognitive task such as match-to-sample elicits stronger and more directional hand preferences than natural behaviors.
Summary
-
We investigated handedness in 10 zoo-living olive baboons during a cognitive task and natural behaviors to evaluate what factors impact handedness in nonhuman primates.
-
During the cognitive task, five baboons were left-handed, and two were right-handed; across five natural behaviors, eight baboons were left-handed, one was right-handed, and one was ambiguously handed.
-
Handedness was stronger during the cognitive task, whereas natural behaviors showed weaker and more varied preferences, suggesting that cognitive complexity impacts hand preferences.
Handedness is when an individual shows a preference for one hand over the other, specifically in tasks where only one hand is needed (Papademetriou, Sheu, and Michel 2005). The human species has a right-hand preference—approximately 90% of individuals are right-hand dominant, and approximately 9% are left-hand dominant (Papadatou-Pastou et al. 2020). Population-level right-handedness in humans is thought to be related to left-hemisphere lateralization for language in the brain, but the causal direction of that relation is unclear (Margiotoudi et al. 2019; Meguerditchian, Molesti, and Vauclair 2011; Molesti, Vauclair, and Meguerditchian 2016). Approximately 90% of right-handed individuals exhibit left-hemisphere lateralization for language. Similarly, around 70% of left-handed individuals also have language lateralized in the left hemisphere of the brain. This indicates that handedness and language lateralization develop independently in brain localization (Meguerditchian, Molesti, and Vauclair 2011), and that the evolution of handedness in humans may have been a separate process from language evolution.
Past research suggests a similar individual preference for hand use in nonhuman primates, particularly the great apes such as chimpanzees, bonobos, gorillas, and orangutans (Hopkins 2006). Hand preferences have also been seen in various monkey species, such as olive baboons (Papio anubis; Meguerditchian and Vauclair 2009; Molesti, Vauclair, and Meguerditchian 2016), ring-tailed lemurs (Lamur catta; Masataka 1989; Regaiolli, Spiezio, and Hopkins 2016), and black-handed spider monkeys (Ateles geoffroyi; Motes Rodrigo et al. 2018). However, population-level hand preferences seem to be less common and instead each species tends to have both left-handed and right-handed individuals (Meguerditchian and Vauclair 2009; Motes Rodrigo et al. 2018). In past studies, several terms have been used to describe preferential hand use (e.g., hand preference, manual specialization, task specialization, and true handedness; see McGrew and Marchant 1994), but these terms are not always used (Margiotoudi et al. 2019; Meguerditchian and Vauclair 2009; Molesti, Vauclair, and Meguerditchian 2016). The term “hand preference,” which describes an individual showing preferential use for one hand in a task (McGrew and Marchant 1994), is most commonly used and is the term used throughout the current study.
1 Task Type and Hand Preferences
Like humans, hand preferences in nonhuman primates can be influenced by a variety of factors. Varying complexities of tasks or gestures will elicit more directional (left-handed or right-handed) and strong (high vs. low handedness strength) hand preferences (e.g., Meguerditchian and Vauclair 2009; Molesti, Vauclair, and Meguerditchian 2016). Tasks can be classified as bimanual or unimanual. Bimanual tasks are tasks that are completed with the use of both hands simultaneously with each hand completing a different but complementary action (Zhao, Gao, and Li 2010). In contrast, a unimanual task is a task in which only one hand is needed to complete the action. Bimanual tasks are typically more manually complex (which require coordinated bimanual hand use; Hopkins 2006) and more challenging for individuals to complete compared to unimanual tasks, and in many instances are better indicators of hand preferences in baboons and other nonhuman primates (Molesti, Vauclair, and Meguerditchian 2016; Motes Rodrigo et al. 2018). A variety of manually complex tasks have been presented to nonhuman primates (Fan et al. 2017; Molesti, Vauclair, and Meguerditchian 2016; Schweitzer, Bec, and Blois-Heulin 2007), but handedness during a cognitively complex task, or a task that requires more cognitive resources (such as memory or attention) but does not require motor precision to complete, has not yet been investigated.
Research on humans has found that cognitively complex tasks have a significant impact on hand preference, and as the cognitive complexity increases, humans tend to increasingly rely on their dominant arm and hand to complete the task independent of the manual complexity (Gabbard, Tapia, and Helbig 2003; Liang, Wilkinson, and Sainburg 2018). This finding indicates that cognitive load, or the extent to which cognitive resources are used during a task that requires problem-solving, thinking, and reasoning (Salahuddin and Ismail 2015), can impact and possibly strengthen the use of the preferred limb in humans, which suggests that we may see a similar effect in nonhuman primates. If there is a strong effect of cognitive complexity on hand preference, it can indicate that evolutionarily, hand preferences may be closely related to the development of advanced cognition functions other than language such as problem-solving and decision-making in nonhuman primates.
Manual tasks have commonly been used to investigate hand preferences in nonhuman primates, and individual hand preferences are frequently found in a variety of different species (Fragaszy and Mitchell 1990; Meguerditchian and Vauclair 2009; Motes Rodrigo et al. 2018; Vauclair and Fagot 1987) but population-level hand preferences are less common (Hopkins 1995; Hopkins, Dahl, and Pilcher 2000; Masataka 1989; Meguerditchian, Molesti, and Vauclair 2011). For example, reaching tasks tend to elicit individual hand preferences in either direction, as reported in olive baboons (Molesti, Vauclair, and Meguerditchian 2016), black-handed spider monkeys (Motes Rodrigo et al. 2018), northern white-cheeked gibbons (Nomascus leucogenys; Fan et al. 2017), and western low land gorillas (Gorilla gorilla gorilla; Lambert 2012). Manually complex experimental tasks, such as a tube task (where a subject uses one hand to hold a tube and the other hand retrieves a food item from inside), show individual hand preferences in both directions in black-handed spider monkeys (Motes Rodrigo et al. 2018), common squirrel monkeys (Saimiri sciureus; Meguerditchian et al. 2012), northern white-cheeked gibbons (Fan et al. 2017), bonobos (Pan paniscus; Chapelain and Hogervorst 2009; Chapelain et al. 2011), and in chimpanzees (Pan troglodytes; Llorente et al. 2011). Other tube task studies found enough individual hand preferences in one direction (left- or right-handed) that there was a population-level hand preference. Olive baboons (Molesti, Vauclair, and Meguerditchian 2016) and chimpanzees (Hopkins 1995; Hopkins, Dahl, and Pilcher 2000) showed population-level right-hand preferences, and De Brazza's monkeys (Cercopithecus neglectus; Schweitzer, Bec, and Blois-Heulin 2007) showed population-level left-hand preferences. All studies utilizing a tube task showed hand preferences to some degree.
When observing natural behaviors rather than an experimental manual task or cognitive task, past studies suggest that subjects will have strong preferences for some behaviors but not others. For example, when De Brazza's monkeys engaged in natural behaviors, there were hand preferences for some behaviors (carrying to the mouth, holding), but not others (Schweitzer, Bec, and Blois-Heulin 2007). Additionally, a troop of bonobos had hand preferences for some natural behaviors, but the strength differed from behavior to behavior. Two behaviors that showed significantly stronger hand preferences were “leading limb in locomotion” and “carrying” (Hopkins et al. 1993). There could be a variety of different reasons to account for the fact that nonhuman primates will exhibit stronger hand preferences for some natural behaviors, but not others. If the behavior is inherently communicative or involves tool use, it may be more likely to elicit a strong hand preference (Colell, Segarra, and Sabater-Pi 1995).
Some studies focus on one specific experimental task, whereas others present multiple tasks to the same sample and compare their preferences. One such study evaluated hand preferences in chimpanzees in a series of four tasks and showed that many of the subjects had a consistent hand preference as they completed each task (Colell, Segarra, and Sabater-Pi 1995). Consistency of hand preferences was also found in several studies that presented subjects with a reaching task and a tube task. Molesti, Vauclair, and Meguerditchian (2016) found consistency of hand preferences across a unimanual reaching task and a bimanual tube task in olive baboons. Motes Rodrigo et al. (2018) presented black-handed spider monkeys with three variations of a tube task as well as a unimanual reaching task and found that a majority of the subjects preferred the same hand across tasks. However, consistency across tasks is not always observed. Fan et al. (2017) observed inconsistent hand preferences in northern white-cheeked gibbons across three different tasks, suggesting that some species may adjust their preferences based on task demands. Consistent hand preferences across tasks indicate that an animal can have stable hand preferences independent of external factors such as the subject's position relative to the object, convenience, or task complexity.
2 The Current Study
Studies on handedness in nonhuman primates tend to focus on either an experimental task (e.g., the tube task) or observations of natural behaviors. The current study investigated the hand preferences in a troop of 10 olive baboons residing at a zoo that participated in a cognitive task on a touchscreen computer and were also observed in their habitat as they carried out their natural behaviors. There have been many studies that have investigated hand preferences in nonhuman primates as they engage in manually complex tasks (e.g., tube task; Hopkins 1995; Molesti, Vauclair, and Meguerditchian 2016; Motes Rodrigo et al. 2018). However, no prior study has examined handedness during a cognitively complex task using a touchscreen computer.
The cognitive task is a match-to-sample game requiring one hand to touch the screen and retrieve a food reward for correct answers. There have been other studies that use touchscreens to present similar tasks but have not observed handedness as the subjects complete the task (Harrison, Mohr, and van de Waal 2023; Perdue et al. 2011). The baboons were engaged in several match-to-sample tasks for approximately 3 years during the current study and had experience with the touchscreen for approximately 1 year before any handedness data collection. Natural behaviors were observed over a period of 1 year, including grooming, foraging, body movements, arm movements, hand movements, and sexual behavior. Each of these categories contained several individual behaviors that the baboons engaged in (Table 1), some of which required one hand (e.g., picking up/eating, wiping, and reaching), and some of which required two hands at the same time (e.g., two-handed mutual grooming).
| Category | Behavior | Description of behavior |
|---|---|---|
| Self-grooming | One-handed self-grooming | Picking through skin or fur on self using one hand |
| Two-handed self-grooming (picking) | Using one hand to clean and pick anything out of skin or fur on self, whereas other hand pushes back fur | |
| Mutual grooming (actor) | One-handed mutual grooming (picking) | Picking through skin or fur on conspecific using one hand |
| Two-handed mutual grooming (picking) | Using one hand to clean and pick anything out of skin or fur on conspecific, whereas the other hand pushes back fur | |
| Foraging | Digging | Breaking up the wood chips or dirt with hands in a cupper position to look for food |
| Sweeping | Moving the wood chips or dirt aside in broad strokes with flat hands to look for food | |
| Picking up/eating | Collecting food from the ground and lifting it toward mouth | |
| Food handling | Any action where the subject is ripping, tearing, or otherwise handling their food before it is lifted to their mouth to eat it | |
| Body movement | Climbing upward | Pulling self upward onto higher ground or platform, the leading hand being the dominant hand |
| Climbing downward | Moving downward onto lower ground and off platforms, the leading hand being the dominant hand | |
| Jumping | Jumping from one place to another | |
| Locomotion | Walking, galloping, or running from one place to another without jumping or climbing | |
| Arm movement | Wiping | One hand is moved across the surface of the muzzle on self |
| Reaching | One or both arms extend forward | |
| Throwing | Using force from the arm to propel something forward into the air | |
| Pushing (object) | Using force from the arm and body to move something forward on the ground | |
| Lifting | Picking up a nonfood object and raising it to a higher position | |
| Hand movement | Gripping | Hands are gripped onto any surface (e.g., fence, net, and structure) |
| Holding | Holding something in hand (e.g., food) | |
| Scratching | Scraping or digging the fingernails across the surface of skin or fur in a specific location on self | |
| Aggression | Grabbing | Reaching out and grabbing onto a conspecific |
| Tail pulling | Grabbing onto tail of conspecific and pulling toward self | |
| Poking | Using one finger to poke conspecific | |
| Mounting | One subject mounting a conspecific without copulation | |
| Other aggression | Any other aggressive behavior behaviors that have not been listed, which include pushing, slapping, biting, etc. | |
| Sexual behavior | Mating | One subject mounting and copulating with conspecific |
| Inspecting genitals | Subject touching or inspecting genitals of self | |
| Cognitive task | Playing task | Sitting at the touchscreen and actively playing the game |
| Observing task | Sitting within 10 feet of screen, fighting, waiting, or observing | |
| No behavior | Inactive | When the subject is stationary, no other behaviors observed |
| Out of sight | The subject cannot be seen | |
| Unknown Behavior | Unknown behavior | Any behavior that was not listed but has been observed |
- Note: This table includes a comprehensive list of all baboon behaviors observed. The five most common observed behaviors were digging, sweeping, picking up/eating, wiping, and scratching, which are shown in bold.
We hypothesized that individual baboons will have hand preferences when completing a cognitive task and engaging in natural behaviors, but there will not be a population-level hand preference (Meguerditchian and Vauclair 2009; Motes Rodrigo et al. 2018). A few previous studies have observed population-level hand preferences in olive baboons. However, the sample sizes were much larger than the current study, so we did not expect to see similar results (Meguerditchian, Molesti, and Vauclair 2011; Molesti, Vauclair, and Meguerditchian 2016). We predicted that we would see stronger and more directional individual hand preferences for the cognitive task than the natural behaviors, based on the findings from Gabbard, Tapia, and Helbig (2003). Also, in accordance with past observational studies on natural behaviors in baboons, we predicted that some individual behaviors being observed would be more likely to elicit a hand preference than other behaviors (Harrison and Byrne 2000). Lastly, we hypothesized that the baboons would have the same hand preference for the cognitive task and the natural behaviors requiring one hand. For example, it is predicted that a baboon that preferred to use their right hand to manipulate the touchscreen would also show an overall right-hand preference for other natural behaviors. Consistency of hand preferences across tasks (both experimental tasks and observed behaviors) was found in several past studies (Colell, Segarra, and Sabater-Pi 1995; Molesti, Vauclair, and Meguerditchian 2016; Motes Rodrigo et al. 2018).
3 Methods
3.1 Subjects
The subjects were 10 olive baboons, housed at the Seneca Park Zoo (Rochester, NY). There were six female and four male baboons between the ages of 13 and 30 that were all in continuous full (group) contact. The range of ages given was the subject's age at the completion of the study. Many of the baboons had familial ties with at least one other baboon in the troop, except for Sabina, a baboon with no familial ties (Table 2). Seven out of the 10 baboons voluntarily participated in the cognitive task (Jefferson, Kalamata, Mansino, Olivella, Pepperella, Pico, and Sabina). Subsets of these subjects have participated in cognitive research utilizing reasoning tasks (Ferrigno, Hughes, and Cantlon 2016; Ferrigno, Huang, and Cantlon 2021) and quantity discrimination (counting) tasks (Barnard et al. 2013; Cantlon et al. 2015; Koopman et al. 2019). This research was approved by the AZA-accredited Seneca Park Zoo and the RIT Institutional Animal Care and Use Committee, adhered to the American Society of Primatologists' Principles for the Ethical Treatment of Nonhuman Primates, and adhered to the legal requirements of the United States.
| Baboon | Sex | Age | Familial relationship | Primate Portal trials | ZooMonitor sessions | ZooMonitor observations |
|---|---|---|---|---|---|---|
| Jefferson | M | 16 | Son of Pimento | 1099 | 32 | 714 |
| Kalamata | M | 16 | Half-brother of Olive Oil | 21,958 | 32 | 600 |
| Mansino | M | 19 | Dominant male, father of Pico and Olivella | 476 | 32 | 702 |
| Olivella | F | 13 | Daughter of Olive Oil and Mansino, half-sister of Pico | 8081 | 32 | 651 |
| Pepperella | F | 16 | Daughter of Pearl, mother of Pico | 52 | 32 | 670 |
| Pico | M | 17 | Son of Pepperella and Mansino | 4589 | 33 | 348 |
| Sabina | F | 17 | No familial relationships | 4643 | 36 | 701 |
| Olive Oil | F | 17 | Dominant female, mother of Olivella, half-sister of Kalamata | N/A | 32 | 615 |
| Pimento | F | 30 | Mother of Jefferson | N/A | 32 | 599 |
| Pearl | F | 24 | Mother of Pepperella | N/A | 34 | 893 |
| M = 5842.57 | M = 32.7 | M = 649.3 | ||||
| SD = 7665.51 | SD = 1.34 | SD = 135.96 |
- Note: A total of 1506 ZooMonitor observations were excluded across baboons. Some behaviors were excluded from analysis (inactive, out of sight, and locomotion) because no hand use was required.
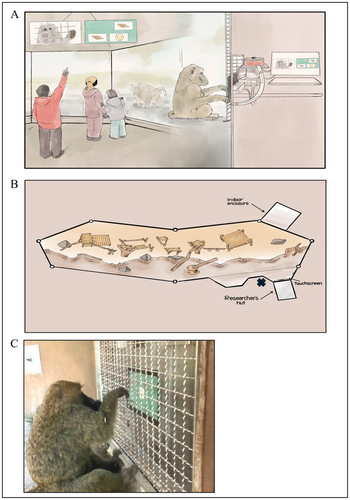
The baboon habitat at the Seneca Park Zoo consists of two areas: one outdoor area for public exhibition and an indoor off-exhibit area. All observations were conducted in the outdoor habitat (approximately 26 m long, 8 m deep, and 4–6 m high). The outdoor habitat contains several wooden structures for the animals to climb, as well as trampoline platforms, swings, and fallen logs (Figure 1). The baboons are given other forms of enrichment in their habitat, including toys of different sizes and colors, cardboard boxes, bags, and other objects.
The baboons' daily diet consisted of 2.38 kg of Mazuri Primate LS Cinnamon Biscuits (5M1S) as well as 1.59 kg of vegetables (e.g., carrots, celery, and broccoli) and fruits (e.g., apples and oranges). They were also given a forage mix, which consisted of a seed mix and two other various food items (e.g., beans, peas, rice, and wheat). The baboons received their entire daily diet regardless of whether they participated in the cognitive task. The daily diet was determined by the animal care staff at the Seneca Park Zoo, and their diet remained consistent over the whole period of data collection. The baboons received banana-, apple-, grape-, and orange-flavored Bio-Serv fruit crunchies (190 mg) as a primary reinforcer for the match-to-sample task on the touchscreen computer (1 pellet per correct answer).
3.2 Materials
3.2.1 Cognitive Tasks
The Primate Portal is depicted in Figure 1A. The Primate Portal laboratory at the Seneca Park Zoo includes a touchscreen computer (Model TSD-45-16R) produced by Teguar (14.5 cm × 19.5 cm) that the baboons have access to engage in cognitive tasks. An automated feeder (ENV-203-190-IR) produced by Med Associated Inc. dispenses food upon the correct answer. The food dispenser is made to deliver 190 mg pellets; however, our feeder has been modified to deliver larger reinforcers. A video camera (Amcrest Outdoor PoE Dome Camera with 4K Ultra HD video) is used for livestreaming and recording the sessions onto the Primate Portal YouTube channel (www.youtube.com/@primateportal3736). Two monitors (SunBrite outdoor TV) showing the stimuli being viewed by the baboons were positioned above the glass viewing windows so that zoo guests could observe the trials in real time. Research assistants were positioned outside in the viewing area on the other side of the glass where the touchscreen was set up to collect handedness data (marked with an “X” in Figure 1B), and another research assistant ran the computer from within the researcher hut. More information about the Primate Portal can be found at https://www.theprimateportal.org/.
3.2.1.1 Stimuli
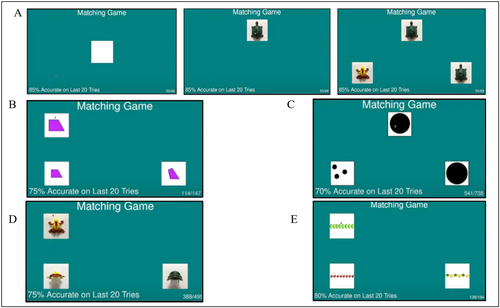
Over the course of a cognitive task session, the subjects were presented with multiple different match-to-sample tasks (e.g., object constancy, geometry, numerosity, and pattern matching). The stimuli were presented to the subjects as 1:1 square digital images (2.54 cm × 2.54 cm) on the touchscreen computer. Example stimuli from each of the tasks and descriptions of each task are shown in Figure 2. Stimulus sets included three to five blocks of stimuli, where each block included trials from one task type (object constancy, geometry, numerosity, or pattern matching) with a total of 718–1592 unique trials in the set. Stimulus sets changed every 3–6 months.
3.2.2 Natural Behaviors
The data collected in the outdoor habitat when observing the baboons' natural behaviors were recorded using a software program called ZooMonitor (https://zoomonitor.org), which was developed by Lincoln Park Zoo to be used for recording animal behaviors (Ross et al. 2016). The data were collected on two Apple iPads (iPad Pro 4th Generation and iPad 7th Generation). The behaviors that were used in ZooMonitor were programmed from a species ethogram that was completed before data collection began (Table 1). To create the ethogram, we followed the focal animal sampling method and recorded every behavior we observed for approximately 10 h over a 2-week period at the same time of day. Research assistants collecting data for the ethogram were not positioned in any specific spot, but rather anywhere in the public viewing area they were able to get a clear view of the subject they were observing at the time. When collecting data, the recorder would often have to follow the subject from one end of the habitat to the other. Figure 3 shows images of baboons engaging in different natural behaviors.

3.3 Procedure
3.3.1 Cognitive Tasks
Cognitive testing sessions at the Primate Portal were typically conducted 3 or 4 days per week in the afternoon for several hours per day. One research assistant (experimenter) was positioned in the researcher hut to run the cognitive task on the computer, and another research assistant (recorder) was positioned in the viewing area making observations of handedness. There were four experimenters and 13 recorders throughout the duration of this study. Two of the recorders were also experimenters. The recorders were trained by two highly experienced research assistants for two sessions (approximately 4 h in total) before collecting data for the study and were always supervised. To ensure consistency, recorders would check reliability with their trainer at the end of their training period by collecting data simultaneously and comparing results. Recorders would only complete training once their data matched the trainers reliably. Data collection for handedness occurred at four steps during each trial (touching the start box, sample stimulus, choice stimulus, and retrieving the food) for each trial (Figure 2A).
Baboons engaged voluntarily with the cognitive task for a few trials or hundreds of trials in a single sitting each day, and the baboons themselves determined which baboon played at any given time. Data collection occurred for approximately a year and 6 months from September 2022 to March 2024. Across all subjects, a total of 40,898 trials were recorded, and the number of trials per individual subject can be seen in Table 2. As shown in Table 2, Kalamata and Olivella played most frequently, whereas Mansino and Pepperella played least frequently.
3.3.2 Natural Behaviors
ZooMonitor was used to conduct all-occurrence focal animal sampling sessions (Ross et al. 2016). Each session was 5 min long, and one baboon was observed for the entire session. During these 5 min, the baboon's behavior, and which hand they used to carry out the behavior, was recorded. Both individual behaviors and bouts were collected depending on the behavior. Every instance of most behaviors was recorded; however, there were some exceptions. For example, the foraging behaviors “sweeping” and “digging” were recorded as a bout, which is measured as when the subject begins to display the behavior and ends when they either switch hands or begin a new behavior. We decided to measure these behaviors as bouts because they occur continuously over a single period and may not lead to accurate counts when scored as individual behaviors.
There were three different recorders who were all trained by the same experienced research assistant. Recorders did not collect data included in the study until they could correctly identify all baboons and all behaviors. The number of sessions and observations for each baboon is listed in Table 2. The following behaviors were excluded from the total number of observations because they do not require hand use: inactive, out of sight, and locomotion. The following behaviors (which occurred in the initial observations but did not typically occur daily) were not excluded from analysis but were very rarely displayed, and we recorded only a few observations or none at all: throwing, pushing (object), lifting, holding, grabbing, tail pulling, poking, other aggression, mounting, mating, and inspecting genitals. Although all behaviors were recorded, the analysis focused on the five most common behaviors that all 10 baboons engaged in: digging, sweeping, picking up/eating, wiping, and scratching.
A reliability check for observing the natural behaviors in the outdoor habitat was conducted by video recording twenty 5-min sessions of active baboons (two sessions for each of the 10 baboons). The videos were recorded using a Kimire video camera (full HD 1080p, 15FPS, 24 MP). Each video was recorded at times when the baboon was actively engaging in behaviors, both in the morning and afternoon, just as if we were recording data live. Two of the three recorders (who recorded 96% of the original study data) coded the video recording on ZooMonitor as if they were watching the session live. The responses from each recorder were then compared by keeping a count of each behavior and whether the behavior was done with the left or right hand. The counts for each behavior and hand were compared between recorders for each session. To calculate the similarity, a formula was used in Microsoft Excel: ABS(1 – ABS[Recorder 1 n] – [Recorder 2 n])/MAX (1[Recorder 1 n], [Recorder 2 n]) × 100, where n is the number of observations recorded for specific behavior. This formula first finds the difference between the two values between recorders, then takes the absolute value of the difference, then determines the larger of the two values between recorders, then divides the absolute difference by the larger value, subtracts the normalized difference from 1, and then multiplies by 100 to create a percentage. Reliability was generally high overall (79.21% agreement on all behaviors across 20 sessions).
3.4 Data Analyses
Hand preferences were calculated using individual z-scores based on the number of left-hand and right-hand uses, utilizing the formula z = (R – N × 0.5)/√(N × 0.25), which was used in previous studies (Lambert 2012; Meguerditchian and Vauclair 2006). The z-scores were used to calculate if the subject had a hand preference for both the cognitive task and natural behaviors, and if so, in what direction. The z-scores were also calculated for each individual behavior to see if some behaviors were more likely to elicit a hand preference than others. These values of the z-scores indicated if the subject was left-handed (z ≤ –1.96), right-handed (z ≥ 1.96), or ambiguously handed (–1.96 < z < 1.96).
The handedness index (HI) score was calculated for each individual baboon to determine the degree of handedness for both the cognitive task and natural behaviors (Molesti, Vauclair, and Meguerditchian 2016; Meguerditchian, Molesti, and Vauclair 2011). The HI scores were also calculated for individual behaviors to see if some behaviors elicited stronger hand preferences than others. The HI was calculated using the formula (R – L)/(R + L), where R is the number of right-hand uses, and L is the number of left-hand uses. The scale was on a continuum of –1.0 to 1.0 where a negative sign indicates a left-hand preference, and a positive sign indicates a right-hand preference. The absolute values (ABS-HI) of the HI scores indicate the strength of the individual hand preference independent of the direction. There does not seem to be a consensus in the literature about where the threshold lies between weak preferences versus strong preferences, and it is unclear how these values are determined. In the present study, a right-hand preference was considered strong if HI ≥ 0.5, and a left-hand preference was considered strong if HI ≤ –0.5. When using the absolute value of the HI score, their hand preference was considered strong if HI ≥ 0.5. The HI scores and z-scores for the cognitive task and natural behaviors were compared to determine the consistency of hand preference.
4 Results
4.1 Cognitive Tasks
4.1.1 Direction of Hand Preference
Table 3 shows the z-scores for the cognitive task hand preference for each individual match-to-sample step (touching the start box, sample stimulus, choice stimulus, and retrieving the food). Based on the z-scores for overall hand use in the cognitive tasks (excluding the food retrieval), five baboons were left-handed, and two baboons were right-handed. Five baboons had a consistent hand preference across the steps (excluding the food retrieval), and two baboons did not have a consistent hand preference across the steps. A one-sample t-test was conducted to determine if the mean z-score across subjects (N = 7) was significantly different from a z-score of 0 (which would indicate no population-level hand preference). The mean z-score across the sample (M = –0.11; SD = 0.81) was not significantly different from a z-score of 0, t(6) = –0.36, p = 0.73. As hypothesized, baboons had individual hand preferences but there was no population-level hand preference.
| Baboon | Primate Portal | Natural behaviors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Start | Sample | Choice | Food (left) | Food (right) | Overall | Digging | Sweeping | Picking up/eating | Wiping | Scratching | |
| Jefferson | –17.35 | 1.75 | –1.54 | –95.60 | –15.87 | 15.99 | –7.92 | –2.39 | –3.31 | –7.57 | –0.77 | –0.50 |
| Kalamata | –140.33 | –145.11 | –80.15 | –17.88 | –77.81 | 99.75 | –4.41 | –0.79 | –2.79 | –4.51 | 2.89 | 0.56 |
| Mansino | –2.49 | –1.29 | –1.56 | –1.47 | –3.25 | 4.79 | –1.32 | –0.58 | 0.54 | 0.64 | –1.98 | –1.30 |
| Olivella | 153.77 | 88.73 | 88.90 | 88.71 | –71.38 | 26.65 | –2.48 | –1.54 | –0.64 | –1.58 | –3.16 | 0.63 |
| Pepperella | –11.12 | –6.67 | –6.30 | –6.30 | –3.32 | 1.70 | –10.75 | –1.39 | –4.84 | –10.06 | –2.22 | –1.58 |
| Pico | –107.92 | –65.91 | –62.18 | –58.85 | –54.85 | 20.21 | –8.49 | –1.53 | –7.67 | –5.03 | –1.21 | 1.61 |
| Sabina | 115.66 | 66.82 | 66.77 | 66.75 | –41.24 | 37.96 | –5.30 | –1.63 | –3.67 | –3.93 | –1.71 | –1.86 |
| Olive Oil | — | — | — | — | — | — | –20.00 | –4.11 | –7.34 | –17.32 | –1.96 | –2.40 |
| Pimento | — | — | — | — | — | — | –7.96 | –3.16 | –1.45 | –9.14 | 1.06 | –0.60 |
| Pearl | — | — | — | — | --- | — | 15.81 | 4.53 | 6.09 | 11.17 | 2.83 | 1.62 |
- Note: z-scores were calculated to determine the direction of hand preference (left-handed, right-handed, or ambiguously handed). Individual z-scores classified a subject as left-handed (z ≤ –1.96), right-handed (z ≥ 1.96), or ambiguously handed (–1.96 < z < 1.96). Only the five most common natural behaviors are shown. Left-hand preferences are indicated in bold, right-hand preferences are indicated in italics, and ambiguously handed subjects are indicated in roman.
4.1.2 Strength of Hand Preference
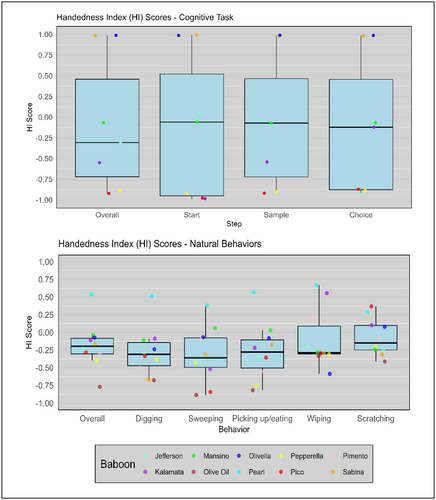
HI scores for each baboon are shown in Table 4 and Figure 4. For overall hand use on cognitive tasks, five baboons had a strong hand preference (HI ≥ 0.5, HI ≤ –0.5) and two baboons had a weak hand preference (–0.5 < HI < 0.5). No baboons had ambiguous hand preferences. Four baboons had a strong hand preference across all steps (Olivella, Pepperella, Pico, and Sabina), Kalamata had strong preferences for all steps except for when he was tapping on the choice stimulus, and Jefferson had a strong preference only when he was tapping on the choice stimulus. As hypothesized, the baboons had strong hand preferences when they engaged in the cognitive task. A one-sample t-test was conducted to determine if the mean HI score across subjects (N = 7) was significantly different from an HI score of 0 (which would indicate no population-level hand preference). The mean HI score across the sample (M = –0.13, SD = 0.89) was not significantly different from a HI score of 0, t(6) = –0.39, p = 0.71. This supports the prediction that there were no population-level hand preferences in this sample.
| Baboon | Primate Portal | Natural behaviors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Start | Sample | Choice | Overall | Digging | Sweeping | Picking up/eating | Wiping | Scratching | |
| Jefferson | –0.31 | 0.05 | –0.05 | –0.92 | –0.23 | –0.29 | –0.40 | –0.34 | –0.20 | –0.25 |
| Kalamata | –0.55 | –0.98 | –0.54 | –0.12 | –0.11 | –0.09 | –0.52 | –0.22 | 0.56 | 0.10 |
| Mansino | –0.07 | –0.06 | –0.07 | –0.07 | –0.03 | –0.11 | 0.06 | 0.03 | –0.28 | –0.24 |
| Olivella | 0.99 | 0.99 | 0.99 | 0.99 | –0.07 | –0.24 | –0.07 | –0.08 | –0.59 | 0.08 |
| Pepperella | –0.89 | –0.92 | –0.90 | –0.88 | –0.40 | –0.38 | –0.43 | –0.75 | –0.31 | –0.21 |
| Pico | –0.92 | –0.98 | –0.54 | –0.12 | –0.28 | –0.33 | –0.84 | –0.36 | –0.29 | 0.37 |
| Sabina | 0.98 | 0.99 | 0.98 | 0.98 | –0.16 | –0.67 | –0.31 | –0.18 | –0.29 | –0.31 |
| Olive Oil | — | — | — | — | –0.77 | –0.68 | –0.88 | –0.82 | –0.33 | –0.41 |
| Pimento | — | — | — | — | –0.31 | –0.50 | –0.10 | –0.55 | 0.19 | –0.09 |
| Pearl | — | — | — | — | 0.54 | 0.51 | 0.38 | 0.57 | 0.67 | 0.29 |
- Note: Handedness index (HI) scores were calculated to determine the strength of hand preference in either direction. Hand preferences were considered strong if HI < –0.5, or HI > 0.5. Only the five most common natural behaviors are shown. Strong hand preferences are indicated in bold.
4.1.3 Correct vs. Incorrect Responses
The direction and strength of hand preferences were calculated for each baboon for the trials where they chose the correct or incorrect stimulus. z-scores (Table 5) show that when choosing the correct answer, four baboons were left-handed, two baboons were right-handed, and one baboon was ambiguously handed. Compared to the overall hand preferences on cognitive tasks, six baboons had a consistent hand preference for overall handedness and correct responses. These hand preferences were equivalent regardless of whether individuals made correct or incorrect responses. Similarly, the strength of the hand preferences for each individual was consistent across correct and incorrect responses. A majority of baboons had a consistent hand preference for overall hand use, as well as for correct and incorrect answers.
| Baboon | Answer | Match-to-sample task | ||||
|---|---|---|---|---|---|---|
| Correct | Incorrect | Numerosity | Object constancy | Geometry | Pattern matching | |
| Jefferson | –12.95 | –11.58 | –11.64 | –7.26 | –11.62 | –1.79 |
| Kalamata | –124.04 | –65.64 | –56.88 | –86.74 | –91.99 | –13.27 |
| Mansino | –1.48 | –2.05 | –3.67 | –0.51 | –2.99 | — |
| Olivella | 133.43 | 76.44 | 78.41 | 98.98 | 87.74 | — |
| Pepperella | –8.57 | –7.10 | –3.46 | –8.98 | –5.34 | — |
| Pico | –96.18 | –49.64 | –53.27 | –67.74 | –61.14 | –12.25 |
| Sabina | 97.09 | 62.86 | 29.17 | 82.51 | 73.35 | 11.09 |
- Note: z-scores were calculated to determine the direction of hand preference (left-handed, right-handed, or ambiguously handed) for accuracy (correct and incorrect responses) and match-to-sample tasks at the Primate Portal (numerosity, object constancy, geometry, and pattern matching). Individual z-scores classified a subject as left-handed (z ≤ –1.96), right-handed (z ≥ 1.96), or ambiguously handed (–1.96 < z < 1.96). Left-hand preferences are indicated in bold, right-hand preferences are indicated in italics, and ambiguously handed subjects are indicated in roman.
To determine if any of the subjects had a side bias for the choice step, we calculated the percentage of correct answers when the correct stimulus appeared on the left side of the screen versus the right side of the screen. The subjects had similar percentages of correct responses on both sides (2%–6% difference in accuracy between the left and right across subjects) except for Jefferson and Pepperella. Jefferson answered correctly 95% of the time when the correct stimulus appeared on the left and only 9% of the time when the correct stimulus appeared on the right, whereas Pepperella answered correctly 73% of the time on the left and 42% of the time on the right. The difference in correct answers on the left versus the right for both subjects indicates a left-side bias when they were touching the screen. Both of these subjects chose to engage in fewer trials of the cognitive tasks than four of the five other subjects and both had a left-hand preference.
To determine if hand preferences were affected by the location of the correct stimulus on the screen, we looked at the percentage of overall trials the correct stimulus was presented on the left side of the screen versus the right side of the screen for each individual subject. All subjects had a 0%–2% difference in stimulus presentation on the left versus the right (e.g., Jefferson was presented with the correct stimulus on the left 49% of total trials and on the right 51% of total trials), except for Pepperella. She was presented with the correct stimulus on the left 53% of total trials and on the right 47% of total trials (6% difference). The location of the choice stimulus was equally balanced on the left and right for each session; however, because the subjects could come and go during any point in the session, it sometimes led to trials not being perfectly balanced, and Pepperella played the fewest number of trials which could explain her larger percentage difference. We found that for each subject, the z-scores show that hand preferences are consistent when the correct stimulus is shown on both sides of the screen, regardless of whether the answer was correct or incorrect (Table 5). If their hand preferences were impacted by the location of the correct stimulus, their overall right- and left-hand use should match the percentage of correct stimulus on the left versus the right, which was not the case. This indicates that hand preferences were not influenced by the location of the correct stimulus on the screen.
4.1.4 Effect of Task on Hand Preference
The direction and strength of hand preferences were also calculated for each of the tasks that they were given (numerosity, object constancy, geometry, and pattern matching). The z-scores (Table 5) indicated that when engaging in the numerosity and geometry tasks, five baboons were left-handed, and two baboons were right-handed, identical to the overall hand use on cognitive tasks. Their hand preferences were also consistent when engaging in the object constancy task, except for Mansino. He was left-handed in all tasks except for object constancy, which he was ambiguously handed. Based on HI scores, most baboons also had strong hand preferences for all tasks. The pattern-matching task was only presented briefly, and some baboons did not participate in any trials in that task.
4.1.5 Correlation Between HI Score and Task Performance
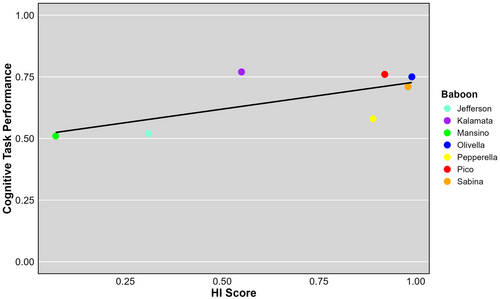
Accuracy across all tasks was collapsed for each baboon because hand preferences were consistent across tasks, except for Mansino, and the mean of the averages was taken for each baboon. Pearson's correlation coefficient was used to assess the relationship between HI score and task accuracy (Figure 5). Absolute values of the overall HI scores were taken to address only strength and not direction. There was a positive correlation between strength of hand preference and task performance (r = 0.7, p = 0.08), which indicates a trend toward a relationship, but it is not statistically significant.
4.1.6 Food Tube Switch
The food reward upon each correct answer was dispensed to a small opening on the left or right side of the baboon below the touchscreen (which side is used is controlled by the experimenters). It can only dispense to one side at a time and was configured to dispense out of the left side from September 2022 to July 2023 (approximately 10 months) and then out of the right side from July 2023 to March 2024 (approximately 8 months). Table 3 shows z-scores for each baboon's hand preference when the food was coming out of the left dispenser and when it was coming out of the right dispenser. When the food was dispensed to the left side, all 10 baboons preferentially used their left hand to remove the food from the opening and bring it to their mouth (z < –1.96). When it was changed to dispense to the right side, all baboons switched hand preferences, nine switching to preferring their right hand (z > 1.96), and one baboon switching to be ambiguously handed (–1.96 < z < 1.96). It is important to note that even though the preferences for the food retrieval changed, the preferences for the first three steps (start box, sample stimulus, and choice stimulus) were stable across the food tube side switch. In addition, most baboons did not show a bias to select images on the left or right side of the screen where the food reward was delivered. When the food was being dispensed to the left side, all subjects except for Jefferson and Pepperella had similar percentages of selecting the correct stimulus on the left versus right side of the screen (0%–3% difference in accuracy across subjects excluding Jefferson and Pepperella), indicating that there were no side biases for these subjects. Jefferson and Pepperella both had left-side biases when the food was being dispensed to the left side (30%–86% difference). When the food was being dispensed to the right dispenser, all subjects except for Jefferson and Pepperella had similar percentages of selecting the correct stimulus on the right versus left side of the screen; however, the range of values was larger (5%–14% difference in accuracy across subjects excluding Jefferson and Pepperella). Jefferson and Pepperella continued to have left-side biases even when the food was being dispensed to the right side (39%–90% difference). This shows that both Pepperella and Jefferson had left-side biases when the food was dispensed to the left side, but this bias remained when the food was dispensed to the right, which indicated that this was not a product of which side the food was dispensed to.
4.2 Natural Behaviors
4.2.1 Direction of Hand Preference
Table 3 shows the z-scores for the hand preferences during natural behaviors for each individual behavior. Based on the z-scores for overall hand use during natural behaviors, eight baboons were left-handed, one baboon was right-handed, and one baboon was ambiguously handed (Table 3). Only one baboon had a consistent hand preference across all behaviors. A one-sample t-test was conducted to determine if the mean z-score across subjects (N = 10) was significantly different from a z-score of 0. The mean z-score across the sample (M = –5.28, SD = 9.07) was not significantly different from a z-score of 0, t(9) = –1.84, p = 0.10, which suggests that there was no population-level hand preference. Across all subjects, a total of 327 sessions and 6493 observations were recorded, and the number of sessions and observations per individual subject can be seen in Table 2. As hypothesized, the baboons had individual hand preferences but no population-level preferences.
4.2.2 Strength of Hand Preference
As hypothesized, the strength of hand preferences was much weaker for natural behaviors than for the cognitive tasks (Table 4 and Figure 4). For overall hand use during the five most common natural behaviors, two baboons had strong hand preferences (HI ≥ 0.5, HI ≤ –0.5), and eight baboons had weak hand preferences (–0.5 < HI < 0.5). A one-sample t-test was conducted to determine if the mean HI score across subjects (N = 10) was significantly different from an HI score of 0. The mean HI score across the sample (M = –0.18, SD = 0.33) was not significantly different from an HI score of 0, t(9) = –1.76, p = 0.11. Across all subjects, a total of 327 sessions and 6493 observations were recorded, and the number of sessions and observations per individual subject can be seen in Table 2. This supports the conclusion that there are no population-level hand preferences in this sample. HI scores for the individual behaviors are presented in Table 4. As predicted, some behaviors elicited stronger hand preferences than other behaviors (Table 4). A subset of baboons had individual hand preferences for each of the five behaviors, except for scratching, which elicited no hand preferences from any individuals. There were not enough individual behavior observations for each baboon to determine if there were population-level hand preferences for specific behaviors.
4.3 Consistency of Hand Preference Between Cognitive Task and Natural Behaviors
There were seven baboons (Jefferson, Kalamata, Mansino, Olivella, Pepperella, Pico, and Sabina) that engaged in both the cognitive task and natural behaviors. Four of these baboons had the same hand preference when they completed the cognitive task and when they were engaging in natural behaviors, and they were all left-hand preferences. The remaining three baboons did not have consistent hand preferences between cognitive task and natural behaviors. Mansino had a left-hand preference for the cognitive task but was ambiguously handed when engaging in natural behaviors. Sabina and Olivella were right-handed for the cognitive task but left-handed when engaging in natural behaviors. Our prediction that a baboon would have the same hand preference for the cognitive task and natural behaviors was only partially supported.
5 Discussion
We assessed the hand preferences in a troop of 10 olive baboons as they manipulated a touchscreen computer to complete a cognitive task, as well as their hand preferences as they engaged in natural behaviors in their outdoor habitat. When the baboons were completing the cognitive task, they had individual hand preferences but not a population-level hand preference, as expected. This is consistent with results from studies that present experimental tasks to their subjects (Meguerditchian et al. 2012; Motes Rodrigo et al. 2018). These results differ from Meguerditchian, Molesti, and Vauclair (2011) and Meguerditchian and Vauclair (2006), who both found population-level right-hand preferences in olive baboons during a “hand slap” behavior.
The cognitive task presented in the current study yields similar findings to other studies with complex manual tasks in that there were strong individual hand preferences. Many other studies that present manually complex tasks to subjects, such as a tube task, have found strong individual hand preferences (Chapelain and Hogervorst 2009; Motes Rodrigo et al. 2018; Schweitzer, Bec, and Blois-Heulin 2007), and even population-level hand preferences (Molesti, Vauclair, and Meguerditchian 2016; Regaiolli, Spiezio, and Hopkins 2016). The task in the current study is distinct because it is cognitively demanding without any physical challenges of manipulation, and the results parallel the findings in similar human studies (Gabbard, Tapia, and Helbig 2003; Liang, Wilkinson, and Sainburg 2018). The similarity between the findings in the current study, which used a cognitively demanding touchscreen task, and findings from previous studies using complex manual tasks, suggests that the cognitive difficulty of the task rather than just the manual difficulty also plays a role in handedness. However, a population-level hand preference might be caused by the type of manipulation in a manual task and does not emerge in a purely cognitive task.
The potential relationship between the HI score and task performance suggests that the strength of handedness is correlated with performance accuracy and is a potential link between cognitive ability and lateralization in nonhuman primates. This supports the findings from Gabbard, Tapia, and Helbig (2003) that cognitively complex tasks have a significant impact on hand preferences and implies that the evolution of hand preferences in primates is linked to the development of more advanced cognition functions, instead of just motor skills. However, it is unknown whether a complex cognitive task is the cause of the strong hand preferences or if individuals with stronger handedness will perform better on cognitive tasks.
When baboons were engaging in their natural behaviors, they had individual hand preferences overall and for individual behaviors; however, they were weak. As predicted, there were no population-level hand preferences. We also expected that some behaviors would elicit more hand preferences than others. There were not enough individual observations per baboon for each behavior to do statistical analyses; however, based on the z-scores, certain behaviors elicited hand preferences in more baboons than other behaviors (e.g., the picking up/eating behavior elicited directional hand preferences in eight baboons compared to the scratching behavior, which elicited directional hand preferences in one baboon).
Past research has also shown that certain natural behaviors elicit stronger hand preferences for others, and some behaviors elicit no hand preferences at all (Forsythe et al. 1988; Meguerditchian and Vauclair 2009). In the current study, six baboons showed a directional hand preference for the “wiping” behavior (two right-handed baboons and four left-handed baboons) out of the 10 baboons observed; however, only three baboons showed strong preferences for this behavior. Another sample of olive baboons also showed weaker hand preferences for wiping behavior (nine right-handed subjects, nine left-handed subjects, and 54 ambiguously handed subjects; Meguerditchian and Vauclair 2009). Findings from both the current study and Meguerditchian and Vauclair (2009) provide evidence that a baboon may not have a dominant hand for certain behaviors and may rely on other factors (e.g., type of gesture, proximity to objects around them) to make a decision about what hand they use. One difference we see from past literature involves behavior that is typically referred to as “hand slapping,” where the baboon will rub or slap their hands on the ground toward either a conspecific or a human (Meguerditchian, Molesti, and Vauclair 2011). In two studies, individual hand preferences and a population-level right-hand preference were found for this behavior (Meguerditchian and Vauclair 2006; Meguerditchian, Molesti, and Vauclair 2011). The nature of this behavior seems to be communicative, as a threatening gesture and could be one reason that the researchers found such strong individual preferences. Interestingly, this is a behavior that is rarely observed in the troop of baboons in the current study. Most other behaviors that have previously been observed in other samples of baboons were also observed in the current study (e.g., muzzle wiping, picking up food, and reaching). We found that these natural behaviors elicited weaker hand preference than more manually or cognitively complex tasks.
As expected, even though hand preferences were weak for natural behaviors, most baboons that participated in both the cognitive task and natural behaviors had a consistent hand preference, or manual specialization (McGrew and Marchant 1994) in both settings. Other studies, such as Motes Rodrigo et al. (2018), who presented three types of tube task, show that hand preferences can remain consistent even if they are not completing the same task. It is worth noting, though, that Motes Rodrigo et al. (2018) used different variations of one task, whereas the cognitive task and natural behaviors in the current study were unrelated.
There did not seem to be any similar hand preferences within family lines. Mansino, Olivella's father, was weakly left-handed at the Primate Portal, whereas Olivella was very strongly right-handed. During natural behaviors, Olivella was weakly left-handed, Mansino was ambiguously handed, and Olive Oil (Olivella's mother) was strongly left-handed. Another family unit that does not share hand preferences is Pearl (mother) and Pepperella (daughter). When they were engaging in natural behaviors, Pearl showed a strong right-hand preference but Pepperella showed a weak left-hand preference.
One factor that did seem to influence hand preference was convenience or the animal's proximity to the object they are engaging with. At the Primate Portal, we had the ability to change which side the food reward was dispensed to for the baboons to retrieve upon the correct answer. We dispensed the food to the left side of the baboons for approximately 10 months, and we found that most baboons had established individual hand preferences when they touched the screen for each of the three steps of the task (start, sample, and choice), but all baboons preferred to use their left hand to retrieve the food reward. We were curious to see what would happen if we started dispensing the food reward to the right side of the baboons instead of the left. After approximately 8 months of data collection with the food dispensing to the right side, we found that although the individual hand preferences when they touched the start, sample, and choice screens stayed the same, the hand they used to retrieve the food reward had changed. Instead of using their left hand to retrieve the food as they had when it was being dispensed on the left side, six out of seven baboons changed their hand preference, but only for the food retrieval. The hand preferences for the first three steps in a trial remained the same for all baboons, but for the food retrieval they had begun using their right hand to retrieve the food instead of their left. These findings relate to the findings of Meunier, Fizet, and Vauclair (2013), which showed that a hand preference can be a factor of convenience rather than being their actual dominant hand, which seems to be the case for the food reward. These findings also demonstrate that their hand preferences for the first three steps are in fact stable hand preferences, and not influenced by convenience. We did not see a bias to tap on the same side of the screen that the food was being dispensed to, regardless of if the stimulus presented was correct or incorrect (e.g., having a bias to tap on the left side of the screen when the food is dispensed to the left even if the response was incorrect). Jefferson tapped on the left side of the screen with his left hand when the food was dispensed to the left regardless of accuracy and also tapped on the left side of the screen with his left hand when the food was dispensed to the right of him. It is possible that he developed a left-side bias because the food was originally dispensed to the left side and then continued to have this bias even when the food was dispensed to the right side.
This study had some limitations. For one, there was a small sample size with the entire troop consisting of 10 baboons. We also lacked equal numbers of sessions and observations across baboons for the natural behaviors because certain baboons tended to be much more active than other baboons in the outdoor exhibit. We were unable to compare hand preferences across the cognitive task and natural behaviors for three of the baboons because they chose not to engage in the cognitive task, two of which had strong hand preferences for most of the behaviors. Lastly, there were a number of factors that could have potentially impacted these results, such as experimental day, reproductive state, consortship, age, injury, initial orientation and position of the baboon to the screen, presence of conspecifics impacting orientation, and so forth, that were not accounted for; however, future research could explore these factors and how they may impact handedness. Factors such as dominance rank or sex were not accounted for due to the small sample size.
There is no other study to our knowledge that observed hand preferences in baboons during a demanding cognitive task. More research would need to be done to support the claim that cognitively complex tasks elicit strong hand preferences like manually complex tasks do (Hopkins 1995). In the future, we could conduct a more manually complex task with our baboon troop, such as the tube task (Hopkins 1995), and compare those hand preferences to those of the cognitive task in the current study. The matching task our subjects completed on the touchscreen computer was not manually complex, yet five out of seven baboons were very strongly handed in either direction. This shows evidence that a cognitively complex task will elicit similar direction and strength of hand preference as a manually complex task. To expand on the question of whether handedness predicts performance or vice versa, we can introduce varying levels of cognitive complexity in a task to examine the effects on both handedness and task performance. There are also theories about how the type of gesture, such as a communicative gesture, can influence and strengthen hand preference (Cochet and Byrne 2013), so we may be able to learn more about their hand preferences in a natural setting if we focus more on specific types of gestures, rather than all behaviors. For example, we could give the baboons a task that requires them to point at something, which has been used as a communicative task in the past (Meunier, Fizet, and Vauclair 2013).
Handedness in nonhuman primates suggests a precursor for handedness in humans. The results from the current study do not support the hypothesis that population-level right-hand preferences in humans evolved from a common ancestral trait. However, the presence of individual hand preferences in nonhuman primates implies an evolutionary basis for lateralization in general (independently of direction). If we learn more about what factors influence hand preference in nonhuman primates, such as types of gestures or tool use (Cochet and Byrne 2013), it may offer some insight into the context of systematic lateralization in the human brain.
To conclude, most of the olive baboons in the current study showed individual hand preferences when they completed a cognitively challenging match-to-sample task. Individual animals showed stronger handedness biases during cognitively demanding tasks than they did during natural behaviors. Unlike humans, however, the direction of hand preferences, right versus left, was inconsistent across individuals. These results suggest that the evolution of a hand preference is independent of the particular direction of the preference in primates.
Author Contributions
Logan R. Brownell: conceptualization (equal), data curation (lead), formal analysis (lead), funding acquisition (supporting), investigation (lead), methodology (equal), visualization (lead), writing–original draft (lead), writing–review and editing (lead). Jessica F. Cantlon: formal analysis (supporting), funding acquisition (lead), project administration (supporting), resources (lead). Caroline M. DeLong: conceptualization (equal), data curation (supporting), formal analysis (supporting), funding acquisition (supporting), methodology (equal), project administration (lead), resources (supporting), supervision (lead), visualization (supporting), writing–original draft (supporting), writing–review and editing (supporting).
Acknowledgments
We would like to acknowledge the Seneca Park Zoo staff, especially General Curator David Hamilton, Superintendent Steve Lacy, and zookeeper Clare Belden. Hugo Angulo from Carnegie Mellon programmed Primate Portal software and designed the hardware rig. We would also like to acknowledge the Rochester Institute of Technology research assistants who helped with data collection: Katie Becker, McKenzie Wolfe, Kera Hampton, Grace Zdrojewski, Issy Hanick-Herman, Joyce Chu, Gabrielle Koehler, Shaya Gibbs, Victoria Curtis, Genevieve Wright, Ava Agusta, Anna Sofia Hege, Samantha Connell, and Jessica Wegman. We thank Emma Winagle and Lucy Ray for the illustrations of the Primate Portal and baboon habitat. Funding for the Primate Portal was provided by National Science Foundation Award No. NCS-2148343 to J.C. Funding for this project was provided by a Rochester Institute of Technology Psychology Summer Undergraduate Research Fellowship to L.B. and a Rochester Institute of Technology College of Liberal Arts Student Research Award to L.B.
Open Research
Data Availability Statement
The data used in this study are available from the corresponding author upon request.




