Route Planning Process by the Endangered Black Lion Tamarin in Different Environmental Contexts
ABSTRACT
Daily, primates take a variety of decisions to establish why, when, and where to move. However, little is known about the factors influencing and shaping primate daily routes. We investigated the decision-making processes linked to route planning in four groups of black lion tamarins (BLT—Leontopithecus chrysopygus). We studied these endangered platyrrhines within four distinct environmental contexts across their natural distribution (i.e., a continuous forest, a 500-ha forest fragment, a 100-ha forest fragment, and a riparian forest). We used the Change Point Test to identify the points of significant direction change (CPs), which can be considered travel goals along BLT daily trajectories and are key components of travel planning. Considering the high importance of fruits and gum in BLT's diet, we predicted that feeding trees would be the main factor shaping their paths (feeding CPs-FCPs). Also, given previous evidence that platyrrhines use landmarks (i.e., characteristic features from the terrain) as nodes in route network systems (i.e., points of intersection connecting habitual route segments), we expected part of CPs to be located close to the intersection points and to be associated with “locomotion” behavior (LCPs). Analyzing 61 daily paths in four forest fragments, our results showed that BLTs planned routes to reach feeding trees, which primarily determined path orientation. As hypothesized, locomotion was the most frequent behavior observed in CPs, but only in the continuous and riparian forests, with LCPs located as close to intersections as FCPs. Interestingly, these two areas presented the most extreme values (i.e., higher and lower values, respectively) in terms of used area, richness of resources and distances traveled between fruit-feeding trees. Our results suggest that BLTs plan daily routes conditional on the environmental context to reach travel goals, likely to maximize route efficiency to reach out of sight feeding trees.
Summary
-
Reaching feeding trees (i.e., fruit or gum feeding trees) is the most important factor driving Black lion tamarins' (BLTs) daily routes across the species' natural distribution.
-
BLTs use landmarks for spatial orientation along daily routes depending on the environmental context, as a strategy to minimize travel cost or as a consequence of forest structure.
-
We bring the first evidence of the use of route network systems by wild BLTs.
Abbreviations
-
- BLT
-
- black lion tamarin
-
- CPT
-
- change point test
-
- CP
-
- change point
-
- FCP
-
- feeding change point
-
- LCP
-
- locomotion change point
-
- OCP
-
- other change point
-
- AKDEc
-
- area-corrected autocorrelated kernel density estimato
-
- FKUD
-
- fixed kernel utilization distribution
-
- MCP
-
- minimum convex polygon
-
- DPL
-
- daily path length
1 Introduction
Primates, when compared to other groups of mammals, stand out for having significantly enlarged brains in relation to their body size, which presumably provides the substrate for their great cognitive abilities and capacity to solve various types of problems (Clutton-Brock and Harvey 1980; Dunbar 2000; Shettleworth 2010; Tomasello and Call 1997). Primate cognitive skills support complex social interactions within and among groups (Dunbar and Shultz 2017), and integrating different sources of information to take efficient decisions regarding territory defense (Willems and Hill 2009), predator avoidance and foraging (Trapanese et al. 2019). However, our understanding of how primates shape their daily paths and the major factors that influence their decision making is still scarce (Holyoak et al. 2008; Janmaat et al. 2021; Nathan et al. 2008; Trapanese, Meunier, and Masi 2018), particularly in disturbed areas, such as small forest fragments or riparian forests. In such environments, the limits of the forest with surrounding matrices (i.e., rivers, roads, crops) or structural changes at forest edges can represent important factors for navigation decision (Noser and Byrne 2014).
Primates' ranging patterns have been investigated in several species dwelling in different kinds of environments, from savannas to tropical forests (Johnson et al. 2015; Reyna-Hurtado et al. 2018; Trapanese, Meunier, and Masi 2018). Among the most important factors discussed in the literature, the availability and distribution of feeding resources have been identified as key drivers of movement for several primate species (Boyer et al. 2006; Garber 1989; Janson 2016; Reyna-Hurtado et al. 2018; Shettleworth et al. 1988), and it also influences grouping patterns, home range size, and daily ranging distances (Albert et al. 2013; Johnson et al. 2015; Ramos-Fernández, Boyer, and Gómez 2006). Food resources fluctuate in time and space at various scales (Janmaat et al. 2016; Riotte-Lambert and Matthiopoulos 2020), challenging primates' capacity to adjust movement and grouping patterns while anticipating the presence of predators and competitors at foraging sites (Lemoine et al. 2023; Garber, Bicca-Marques, and Azevedo-Lopes 2009; Sobral, Fuzessy, and de Oliveira 2023). To reduce the cognitive cost of foraging; that is, the information involved in the cognitive process of decision-making associated with movement (Bertolani 2013), previous studies have suggested that specific terrain features might be used as landmarks (e.g., river bodies, hills, structural changes in the vegetation, important feeding sites or territory borders), which can be seen from large distances and whose locations are memorized and consequently repeatedly used as beacons for spatial information (Asensio et al. 2011; Dolins 2009; Garber 2000; Garber and Porter 2014; Noser and Byrne 2007). By using spatial features of the landscape for orientation, several species of primates develop route network systems composed of route segments that are repetitively traveled through, enabling an effective locomotion across the home ranges to reach daily goals (Abreu et al. 2021; de Guinea et al. 2019; Di Fiore and Suarez 2007; Hopkins 2011; Presotto and Izar 2010; Presotto et al. 2018).
Interpreting primate travel routes and their underlying planning processes (i.e., their ability to anticipate future locations and plan trajectories accordingly to efficiently navigate the environment to reach important locations) represent significant challenges for researchers (Byrne et al. 2009; Janmaat et al. 2021). To reduce subjective results, the change point test (CPT) (Byrne et al. 2009) detects significant changes in direction (change points—CPs) along animal trajectories that can be used to infer where and when travel decisions are made. These CPs can be interpreted as the locations at which primates direct their trajectories and allow the identification of when these events occur along their routine/travel path (Byrne et al. 2009). The travel goals of primates can be categorized based on whether they occur at the CP, with individuals changing direction after reaching an objective to pursue another, or after the CP, where they change direction significantly without stopping to move, usually due to perceiving landmarks or other important visual cues leading to their final destination. Therefore, CPs can be broadly interpreted as: (1) traveling goals (i.e., reaching a specific feeding site, fighting a conspecific group or monitoring determined regions of the home range) and (2) reference points for primates to reach their goals (i.e., important landmarks for spatial orientation, such as hills or river bodies) (Byrne et al. 2009; Noser and Byrne 2014). By identifying the factors associated with route planning in wild primates, we can better understand interspecific variations in spatial cognition, assess how environmental features influence movement, and verify the existence of key resources (Ban et al. 2016; Cunningham and Janson 2007).
In the Central and Southern Americas, primate movement has mainly been investigated in continuous forests, with few studies in fragmented and/or degraded habitats (Trapanese, Meunier, and Masi 2018). The Brazilian Atlantic Forest is known for presenting a high degree of fragmentation due to lasting human activities (Solórzano, Brasil, and de Oliveira 2021). In fact, only about 23% of its original extension is still preserved, mostly in the form of fragments smaller than 50 ha and without legal protection (Jorge et al. 2013; Vancine et al. 2024). Small forest fragments are known for presenting higher abundances of successional tree species and a reduction not only of canopy size, but also of both large fruiting tree density and diversity (Arroyo-Rodríguez and Mandujano 2006; Laurance et al. 2006). Changes in plant species composition due to fragmentation processes are known to induce primate species to rely less on the consumption of fruits from native trees and, consequently, compensate the acquisition of daily calories with the ingestion of other food items, such as animal prey or fruits from nontree growth forms, including lianas and palms, which are more abundant in altered forest habitats (Bicca-Marques, Chaves, and Hass 2020; Chaves, Stoner, and Arroyo-Rodríguez 2012; de Luna et al. 2017; Donati et al. 2020; Irwin 2008; Tutin 1999). Moreover, diversity and distribution of feeding resources are likely to influence primates' movement and feeding patterns. Indeed, there is a tendency for primates to travel longer distances daily and spend more time per feeding bout in areas where resources are more diverse and unevenly distributed (Boyle et al. 2009; Reyna-Hurtado et al. 2018), while low resource diversity and dense homogeneous distribution across the area may induce primates to move short distances between feeding sites of the same species daily (Reyna-Hurtado et al. 2018; Serio-Silva and Rico-Gray 2002).
There are 27 native primate species in the Atlantic Forest, of which 20 are endemic and most are restricted to small forest fragments (Culot et al. 2019; Rylands and Mittermeier 2024). Among the primate species threatened by habitat disturbance, the black lion tamarin (BLT) (Leontopithecus chrysopygus), endemic to the state of São Paulo, draws attention, with an estimated wild population of 1600 individuals (Rezende et al. 2020). Besides its largest wild population at Morro do Diabo State Park (Rezende et al. 2020), and the recent described occurrence at the Carlos Botelho State Park (Moraes Rodrigues, Gagetti, and Piratelli 2016), the species is mostly distributed in small fragments and riparian forests along the interfluve of the Tietê and Paranapanema rivers (Culot et al. 2015; Garbino, Rezende, and Valladares-Padua 2016). Considered Threatened according to the IUCN Red List of Threatened Species (Rezende et al. 2020), the BLT is a small sized primate (ranging from 400 to 700 grams), highly territorial (Peres 1989), with around 70% of the diet represented by fruits and up to 22% represented by gum (Passos 1999; Valladares-Padua 1993), and known to travel around 2000 meters per day (Keuroghlian and Passos 2001; Valladares-Padua 1993).
Given that BLTs currently occur primarily in highly contrasting human-modified forest habitats (Culot et al. 2015; Garbino, Rezende, and Valladares-Padua 2016), which might present different characteristics known to influence primate behavior and movement, such as size and shape of the forest (Arroyo-Rodríguez and Mandujano 2006, 2009; Bicca-Marques, Chaves, and Hass 2020), richness and distribution of resources (Ban et al. 2016; Reyna-Hurtado et al. 2018), and density of conspecifics (Sobral, Fuzessy, and de Oliveira 2023), here we investigate the route planning process of BLTs in distinct contexts along the natural distribution of the species. We sampled and compared BLTs' behavior and movement between a continuous forest, a medium forest fragment, a small forest fragment, and a riparian forest. Considering the high importance of fruits and gum in BLT's diet (Passos 1999; Silva 2022), we hypothesized that feeding trees (i.e., fruit feeding trees and gum feeding trees) would be the principal factor shaping their trajectories, independently of the area. Consequently, we predicted that most of the CPs would correspond to feeding trees (feeding CPs—FCPs) in all study sites. We also expected to have a higher proportion of FCPs, relative to the frequency of behaviors observed, as most routes directed toward feeding trees would culminate in a FCP. Also, considering the previous evidence of platyrrhines using landmarks and habitual route segments to orient their daily routes (Abreu et al. 2021; Garber and Porter 2014; Presotto et al. 2018), we hypothesized that BLTs would use intersection points of routes as locations for spatial reorientation while traveling between goals further along their routes. Thus, we expected a larger number of CPs to occur near these intersections, but primarily when CPs occurred during locomotion (locomotion CPs—LCPs). In this context, intersections would serve as key points for spatial reorientation, allowing BLTs to adjust their direction effectively as they navigate toward their distant goals.
Finally, it is known that larger and more preserved forest areas present higher richness of fruit species, larger trees, and fewer constraints for primate movement (Arroyo-Rodríguez and Mandujano 2006; Laurance et al. 2006). In contrast, in smaller areas, BLTs; travel paths are constrained by the physical limits of the forest. In such contexts, with limited fruit richness and spatial constraints, shorter travel paths might be sufficient for the groups to access all available nutrients across the areas, even if they do not lead to preferred feeding sites or allow BLTs to meet their full nutritional needs. In larger and more preserved areas, traveling longer distances to reach preferred feeding trees might be associated with higher cognitive costs due to the memorization of a larger number of points for spatial reorientation along the trajectories (Porter and Garber 2013). Therefore, we hypothesized that the use of landmarks is linked to the cognitive costs associated with memorizing the location of out-of-sight feeding resources. To assess this hypothesis, we first examined if BLTs have larger areas used in the continuous forest in comparison to the fragments due to higher habitat availability. Also, considering habitat availability, we tested if BLTs travel longer distances between successive fruit feeding sites in the continuous forest than in the forest fragments. If they do, we predicted that BLTs would recur to the use of CPs for spatial reorientation more frequently in the continuous forest compared to the fragments.
2 Methods
This study adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Nonhuman Primates and followed the American Society of Primatologists' Code of Best Practices for Field Primatology. Permits to study BLTs were provided by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), Ministry of the Environment (Sisbio processes #41375, #43607, #65113, #68253), the Comissão Técnico-Científica do Instituto Florestal (COTEC; N°153/2021 D28/2021PH), and by the Animal Use Ethics Committee (CEUA—IB, UNESP, Rio Claro: #6581).
2.1 Study Areas
Four BLT groups inhabiting four different forest fragments were studied. The first one, situated in the far west of the state of São Paulo, in the municipality of Teodoro Sampaio, the Morro do Diabo State Park (hereafter called “continuous forest”; 22° 37’ S, 52° 10’ W) is one of the largest inland seasonal semi-deciduous Atlantic Forest remnants (Adams et al. 2008). The continuous forest comprises 33800 ha of forest and is home to the largest wild BLT population, estimated around 1200 individuals (Rezende et al. 2020). The second study area is a 505 ha private forest fragment called San Maria farm (hereafter called “medium fragment,” 22°14'5.280“S, 52°18'8.640“W), located in the municipality of Presidente Epitácio, 40 km North of the continuous forest. During the study period, there were three groups of BLTs sharing the fragment. Our third study site, 400 km distant from the continuous forest, is a 100-ha private forest fragment belonging to Santo Antônio farm, in the municipality of Guareí (hereafter called “small fragment,” 23° 25’ 07” S, 48° 14’ 27” W), where three groups of BLTs share the fragment and have frequent intergroup contact. Finally, our fourth study site is a private riparian forest fragment owned by the enterprises Suzano S. A. and Duratex S. A. in the municipality of Lençóis Paulista (hereafter called “riparian forest; 22° 45’ 43.6” S; 48° 59’ 43.1” W). The riparian forest is 330 km distant from the continuous forest and 100 km distant from the small fragment and is mainly surrounded by Eucalyptus spp. plantations. Atlantic Forest in this area is limited to other riparian forests along the Rio Claro river and to the Private Reserve Olavo Egydio Setúbal, totaling around 1799 ha. During the study period, there was no contact between the groups of BLTs registered in the area. All areas are characterized by hot humid summers and dry winters. Mean annual temperatures range between 20.9°C and 21.8°C, and annual rainfall between 1100 and 1330 mm (Alvares et al. 2013; Faria and Pires 2006) (Figure 1).
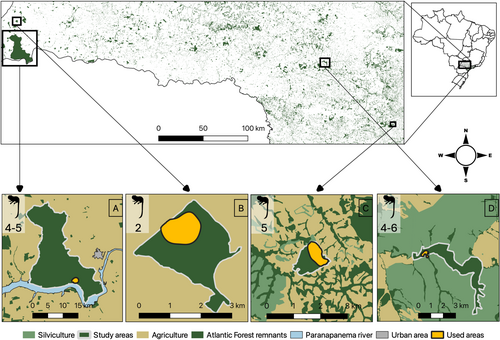
2.2 Data Collection
To collect data on BLT daily routes, we sampled one group of BLTs in each study area during its whole activity period, from the moment the groups left the sleeping site in the morning, until the moment they entered a sleeping site at the end of the day (Coimbra-Filho 1977; Kappeler 1998). In the continuous forest, the group was sampled from December/2017 to September/2018 for a total of 125.7 h distributed along seven entire days (84.1 h) in the wet season and 4 days (41.6 h) in the dry season (Supporting Information S1: Table S1). The group in the medium fragment was sampled from March/2015 to May/2015 during a total of 104 h distributed along 5 days (57.8 h) in the wet season and 6 days (46.2 h) in the dry season. The group in the small fragment was sampled between May/2019 and August/2019 for a total of 233.1 h distributed along 26 days in the dry season. Finally, the group in the riparian forest was sampled for a total of 123.5 h, from September/2018 to April/2019, distributed along 8 days (77.2 h) in the wet season and 5 days (46.3 h) in the dry season (Supporting Information S1: Table S1). We considered the wet season to occur from October to March and the dry season from April to September (Morellato and Haddad 2000; Oliveira-Filho and Fontes 2000). During the sampling period, the group in the continuous forest comprised four to five individuals, of which four were adults; in the medium fragment, the group consisted of an adult couple; in the small fragment, the group included five adult individuals; and in the riparian forest, there were between four and six individuals, of which four were adults. BLT groups in the continuous forest and the medium fragment were already habituated by researchers from both the Instituto de Pesquisas Ecológicas (IPÊ) and from the Laboratory of Primatology from the São Paulo State University (LaP), who had already been conducting long term studies with BLTs in the areas before data collection for the present study. The groups from the small fragment and riparian forest were habituated by the authors to allow data collection for the present study. The habituation process involved locating and actively following the groups for as many hours as possible, for periods ranging from two to 10 consecutive days per month, over three consecutive months in the riparian forest and seven consecutive months in the small fragment. The groups were considered habituated once the individuals stopped fleeing or showing signs of distress in response to the researchers' presence. We did not use individual recognition in the present study.
While sampling the groups, we recorded their location every 5 min using a Garmin GPSMAP 64S device. We characterized each spatial point according to the main activity performed by the individuals of the group. The idea was not to establish an activity budget of BLTs, but to identify how BLTs used specific sites of their used areas. Therefore, using scan sampling method (Altmann 1974), we recorded the main behavior of the group every 5 min. Group activity consisted in the behavior evidenced by the largest number of individuals at sight during the scan. BLTs form cohesive groups, with individuals presenting coordinated activities, rarely distancing themselves from each other (Sussman and Kinzey 1984; Valladares-Padua 1993). We categorized the activities as follow: (1) frugivory = actively searching for, handling, or ingesting fruits; (2) locomotion = any kind of movement through the home range, excluding movement within the same tree; (3) resting and social activities = lying down or engaging in social interactions such as grooming or play; (4) faunivory = actively searching for, handling, or ingesting animal prey; (5) gummivory = ingestion of tree gum; (6) vigilant = remaining stationary, upright, with eyes open, possibly vocalizing; (7) fur-rubbing = rubbing the body against tree trunks to collect balsam; 8) encounter = displaying agonistic vocalizations toward individuals from other groups; (9) long calls = characteristically vocalizing, often to mark territory; (10) scent marking = rapidly rubbing pelvic and thoracic glands against environmental structures; and (11) unknown = behavior could not be assessed due to lack of visibility. The behaviors long call, encounters, scent marking, fur-rubbing, and unknown consisted of events with much shorter duration and consequent lower probability of being recorded in scans than state behaviors (Altmann 1974). To account for this difference, and avoid underestimation of the occurrence of such behaviors, we grouped them in an “other behaviors” category. We recorded the occurrence and duration of all feeding events with the “all occurrence sampling” method (Altmann 1974) during the whole BLTs' activity period. All feeding plants were marked with individual tags in the field, identified to the species level and had the position recorded with a GPS device.
2.3 Data Analysis
We performed all analyses on daily trajectories considering only full-day follows in which we did not lose the groups for more than one scan, which corresponded to 61 out of the 117 days in which we observed habituated BLTs in the field. Therefore, we used a total of 11 full days, with a mean duration (i.e., mean length of activity period of BLTs) of 11.42 h (± SD 1.34 h) for the continuous forest; 11 full days, with a mean duration of 9.45 h (± SD 0.78 h) for the medium fragment; 26 full days with a mean duration of 8.97 h (± SD 0.76 h) for the small fragment; and 13 full days with a mean duration of 9.50 h (± SD 0.79 h) for the riparian forest (Supporting Information S1: Table S1). We defined a step as the Euclidean distance covered between two successive GPS positions (Benhamou 2004), which were captured through scan sampling every 5 min. Also, we defined a travel path, or trajectory, as the sequence of steps used by BLTs to travel through the area (Janmaat et al. 2021; Milton 2000; Trapanese, Meunier, and Masi 2018), and we considered routes to be travel paths reused and connected to other reused paths by nodes in a route network system (Di Fiore and Suarez 2007; Poucet 1993; Trapanese, Meunier, and Masi 2018). If a reused path was not connected to other reused paths, it was not considered a route. We estimated GPS error once a day during data collection in each area using the Precision Indicator from the Garmin GPSMAP 64S device, which showed an estimated positional error ranging between 8 and 12 m overall. To avoid pseudo-movements and extreme relative turning angles due to GPS noise when the groups were stationary (i.e., resting or feeding in the same tree), we considered successive relocations that were within the estimated GPS error when groups were stationary as a single location (Hurford 2009). We estimated daily path lengths (DPL) from the sum of the distances traveled between successive location points recorded during each day.
2.3.1 Estimation of Used Areas
To examine if BLTs have larger used areas in the continuous forest compared to the fragments, and accounting for autocorrelation in current GPS tracking data (i.e., consecutive GPS locations are temporally and spatially correlated with previously recorded locations for the groups) (Noonan et al. 2019), we estimated 95% utilized areas and 70%–75% core areas using the area-corrected autocorrelated Kernel Density Estimator (AKDEc) with the ctmm package version 1.1.0 (Fleming and Calabrese 2017; Fleming et al. 2015). The AKDE is a robust method to consider varying sample sizes as it accounts for autocorrelation in animal movement data, reducing biases that typically arise with smaller datasets and enabling more reliable estimations across different sample sizes (Fleming et al. 2015; Noonan et al. 2019; Silva et al. 2022). We opted for using 70%–75% instead of the more commonly used 50% of Utilization Distribution (i.e., the frequency of use of a certain area) (Winkle 1975) for the estimations of core areas after comparing the observed space-use pattern with that expected for a uniform pattern of use (Vander Wal and Rodgers 2012). We found that these values corresponded to the portions of the used areas in which BLTs exceeded an equal-use pattern (Supporting Information S1: Figure S1) (Rezende 2022; Samuel, Pierce, and Garton 1985). Finally, to avoid the estimations of used areas and core areas to exceed the borders of the forest fragments, we used the shapefiles of the areas as hard boundaries in the AKDEc estimations (Noonan et al. 2019). We also provide 50% AKDEc used area estimations, as well as estimations with traditional methods for comparison (i.e., Fixed Kernel Utilization Distribution—FKUD, and Minimum Convex Polygon—MCP) using the packages adehabitatHR version 0.4.21 (Calenge 2006) and spatstat version 3.0.6 (Baddeley and Turner 2005) (Supporting Information S1: Table S2). We used the previously reported set of data for the estimations of used areas, totaling 1497 GPS locations in the continuous forest; 1236 locations in the medium fragment; 2772 GPS points in the small fragment; and 1469 GPS locations in the riparian forest (Supporting Information S1: Table S1).
2.4 Statistical Analyzes
2.4.1 Route Directionality—Change Points
To understand what goals drove BLT trajectories in the four areas studied, we tested the directionality for the 61 daily trajectories using the CPT method, which is an objective, reproducible method to detect statistically significant turning points along BLTs' daily paths (Byrne et al. 2009). We applied the test backwards on daily paths, analyzing sequentially segments of each trajectory, from the last sleeping site until a CP was detected. This CP then became the starting point for the test to run again and so on until detecting the first CP of the daily path. The CPT verifies if a group of segments after a specific location (i.e., path) is aligned to the path preceding it. It is applied backwards to better account for the significance of important locations for the animals. By doing so, the CPT provides a better quantification of the amount of time an animal had been headed towards a specific location (i.e., the length of the path before a given CP) (Byrne et al. 2009). We tested the number of vectors (q value, from 1 to 10) to be considered on each segment before the detection of each CP to choose the best q value. The best q value is the one allowing the identification of the greatest number of CPs. In our study, with a test sensitivity of p < 0.01, it was equal to 5 in the continuous forest, 5 in the medium fragment, 6 in the small fragment, and 6 in the riparian forest (Supporting Information S1: Figure S2) (Byrne et al. 2009). Finally, to assess whether FCPs represented the highest proportion relative to the frequency of behaviors observed, we calculated CP Ratios for each area. These Ratios indicate the proportion of each CP type relative to the frequency of the corresponding behaviors in our scans. The CP Ratios reflect the extent to which each behavior is preceded by a directed route (Noser and Byrne 2014).
2.4.2 Use of Landmarks
To test if BLTs use points of intersection between routes as points of spatial orientation, we visually identified all locations where at least two routes crossed each-other. Considering that the visual range of platyrrhines spans from 35 to 50 m (Miguel de Guinea et al. 2021; de Guinea et al. 2021; Hopkins 2011; Presotto et al. 2018), we opted to minimize overestimation of route intersections by specifically choosing points of intersection that were a minimum of 35 m apart from each other (Supporting Information S1: Figure S3). We then estimated the distances between each CP and the closest route intersection. To test if CPs associated with feeding trees are more frequently located closer to route intersections, we grouped Gummivory CPs and Frugivory CPs into Feeding CPs (FCPs). We also grouped other CPs not associated to either locomotion or feeding in fixed locations (i.e., Encounter CPs, Faunivory CPs, Fur-rubbing CPs, Vigilant CPs, Long-call CPs, Resting CPs, and Unknown CPs) into the category Other CPs (OCPs) for the following analysis. Using the function glmer, R package lme4 version 1.1.33 (Bates et al. 2015), we fitted Generalized Linear Mixed Models (GLMMs) with a Poisson family distribution and a log link function to explore the association between the number of CPs (response variable) and both the distance to intersections, grouped into categories of 20 m, and the type of CP (i.e., FCPs, LCPs, and Other) (i.e., fixed predictor variables). We selected distance categories of 20 m to remain above the GPS error (8–12 m) while staying below the visibility distance for primates (35–50 m). We standardized continuous variables using the function scale in base R (R Core Team 2023) to improve model convergence and allow comparisons between effect sizes and areas (Schielzeth 2010). Using the full model with all fixed effects, we first selected the most appropriate random effect structure to account for the repeated sampling design (Pinheiro and Bates 2000) by comparing a model with a random intercept by area to models including also a random slope for distance (comparing also both correlated and uncorrelated random slopes), using the Akaike Information Criterion (AIC) (Supporting Information S1: Table S3). We did backwards model simplification on the fixed effects using the function drop1 (R package lme4) to select the best model. Following Jang et al. (2019), we then used single-term deletions and likelihood ratio tests (χ2; function ANOVA, base R, “LRT” test method) from the selected best model to test the significance of each term in the model (Supporting Information S1: Table S4). We checked model assumptions, including overdispersion, using the DHARMa 0.4.6 R package (Hartig 2022) for residual diagnostics of mixed-effects regression models. Model assumptions were reasonably well met.
2.4.3 Distance Traveled Between Fruit-Feeding Trees and CPs
To test if BLTs in the continuous forest travel longer distances between successive fruit-feeding trees, as well as if they recur to the use of CPs for spatial reorientation more frequently than in the fragments, we compared among study sites the distances traveled and the number of scans between consecutive CPs and consecutive frugivory events using one-way ANOVA tests followed by Tukey's HSD post-hoc tests with Tukey correction (functions anova_test and tukey_hsd, respectively, from package rstatix version 0.7.2) (Kassambara 2023). In all models, we used Box-Cox power transformations (function boxcox, R package MASS version 7.3.58.2) (Venables and Ripley 2002) to select the best power transformation of the response variable to meet model assumptions.
All data analyzes were performed using the R Environment for Statistical Computing, version 4.2.3 (R Core Team 2023). All significance values in test, besides the CPT, were set at α ≤ 0.05.
3 Results
3.1 Used Area, Daily Path Length, and Group Behavior
We observed the black lion tamarin (BLT) groups for 61 entire days, totaling 586.2 h, and registered a total of 6974 location points (Supporting Information S1: Table S1). The used areas by the groups varied substantially, in relation also to the total forest size, ranging between 325.8 ha in the continuous forest and 19.4 ha in the riparian forest (i.e., a 16.8 times difference in forest size). Similarly, BLTs traveled longer distances per day in larger areas, ranging from a mean of 3198.5 m (± SD 870.9 m) daily in the largest continuous forest area, to a mean daily path length (DPL) of 894.3 m (± SD 205.8 m) in the riparian forest (Table 1).
| Study site | Area (ha) | 95% AKDEc (ha) | 70%–75%—AKDEc (ha) | 50%—AKDEc (ha) | DPL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Estimate | High | Low | Estimate | High | Low | Estimate | High | Mean ± SD | ||
| Continuous forest | 33845 | 187.3 | 325.8 | 502.4 | 95.9 | 166.4 | 256.4 | 50.3 | 87.4 | 134.6 | 3198.5 ± 870.9 |
| Medium fragment | 494 | 58.7 | 100. 1 | 153.1 | 34.3 | 58.6 | 89.1 | 23.9 | 33.9 | 45.6 | 1897.5 ± 514.7 |
| Small fragment | 100 | 26.7 | 37.9 | 51.0 | 15.8 | 22.5 | 30.3 | 10.1 | 14.4 | 19.4 | 1418.8 ± 313.4 |
| Riparian forest | 1799† | 10.8 | 19.4 | 31.1 | 5.2 | 9.3 | 14.7 | 3.0 | 5.3 | 8.4 | 894.3 ± 205.8 |
- Note: We indicate the 95% confidence intervals (high, low), and highlight the estimated values for the AKDEc estimations. For the continuous forest and the medium fragment, core areas correspond to 75% of the total used area, while for the small fragment and the riparian forest, core areas correspond to 70%. DPL was calculated as the sum of all consecutive steps in each day.
- a Estimated area of the remaining riparian forests along the Rio Claro River and the Private Reserve Olavo Egydio Setúbal in the municipality of Lençóis Paulista.
Frugivory was among the three most frequently sampled group behaviors in all areas, and the most frequent behavior sampled in the scans of both the medium and the small fragments (n = 386/1236 scans; and n = 894/2772 scans, respectively). In the medium fragment, the group was vigilant in 254 out of 1236 scans and resting corresponded to the third most frequent behavior (n = 175/1236 scans). In the small fragment, the second most frequent behavior was faunivory (n = 789/2772 scans), and the third was locomotion (n = 675/2772 scans). In the continuous forest, the most frequent behavior recorded was locomotion (n = 561/1447 scans), followed by resting (n = 329/1447 scans) and frugivory (n = 227/1447 scans). In the riparian forest, the group was resting in 471 out of 1469 scans, moving in 394 out of 1469 scans, and consuming fruits in 268 out of 1469 scans (Supporting Information S1: Table S5).
For all areas, we registered a total of 584 frugivory events on 462 plant individuals belonging to 45 species and 23 families. The highest richness of fruit species consumed by BLTs was recorded in the continuous and riparian forests, while the lowest richness was observed in the small fragment. We report fruit species richness, number of feeding trees, number of visits and the contribution of main species for each of the study areas as Supporting Information (Table S6, Table S7).
3.2 Route Directionality—Change Points
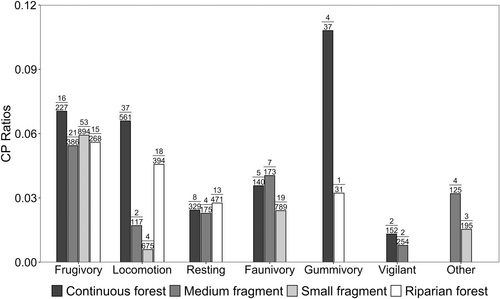
Using the CPT method, we identified a total of 238 CPs, with a mean of 6.5 (± SD 2.0) CPs per day in the continuous forest; 4.0 (± SD 0.9) CPs per day in the medium fragment; 3.0 (± SD 1.1) CPs per day in the small fragment; and 4.0 (± SD 1.8) CPs per day in the riparian forest (Figure 2). Frugivory was the most frequent type of CP in the medium and small fragments, while Locomotion CPs were the most frequent in both the continuous and the riparian forests (Figure 2, Supporting Information S1: Table S5). When looking at the ratio of each type of CP in relation to scans (i.e., the importance of each behavior for directing BLT's routes), Frugivory CPs presented the highest values in all areas but the continuous forest, where Gummivory CPs corresponded to the most important type of CP. Locomotion CPs were the second most important type of CP in the riparian forest while almost tied with Frugivory CPs as second most important in the continuous forest (Figure 3).
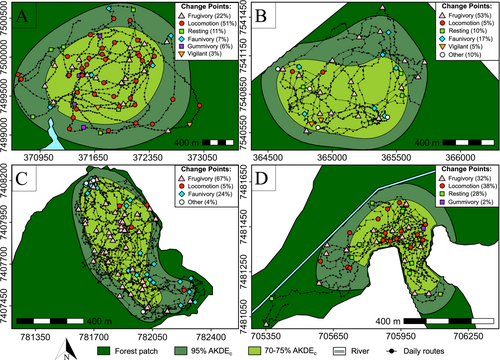
3.3 Route Networks and CPs
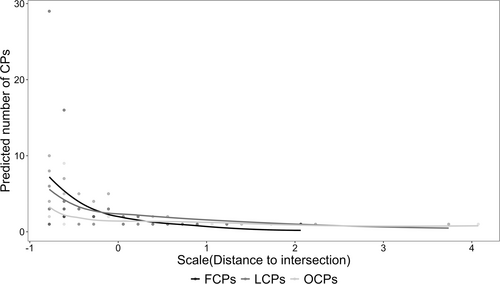
The distance to intersections, the type of CP, and the interactions between the distance and the type of CP significantly affected the number and hence location of CPs (p < 0.05, Figure 4; Supporting Information S1: Table S4). Specifically, we found FCPs to be located closer to intersections and this effect was stronger than for the other types of CPs (Table 2). LCPs were distributed marginally farther from intersections than FCPs, whereas OCPs were located significantly farther from intersections when compared to FCPs and LCPs in all areas (Figure 4, Table 2). We found the distances between CPs and intersections to be lower in the small fragment, followed by the riparian forest, continuous forest, and medium fragment (Supporting Information S1: Table S8).
| Effect | Estimate | SE | CLlower | CLupper | z value | p |
|---|---|---|---|---|---|---|
| Intercept (FCPs) | 0.725 | 0.157 | 0.417 | 1.033 | 4.613 | < 0.001 |
| Distance to intersection (FCPs) | −1.459 | 0.431 | −2.304 | −0.614 | −3.385 | < 0.001 |
| LCPs | 0.188 | 0.210 | −0.224 | 0.599 | 0.893 | 0.372 |
| OCPs | −0.310 | 0.216 | −0.733 | 0.113 | −1.436 | 0.151 |
| Distance to intersection:LCPs | 0.598 | 0.313 | −0.015 | 1.211 | 1.913 | 0.056 |
| Distance to intersection:OCPs | 0.878 | 0.301 | 2.915 | 0.288 | 1.468 | 0.003 |
- Note: CLlower and CLupper indicate the lower and upper confidence limits for the estimated parameters, respectively. The z-value, also known as the Wald statistic, measures the significance of each predictor variable's contribution to the model, with statistical significance represented by the corresponding p-values. Single-term deletions using likelihood ratio tests (χ2) were conducted to evaluate the contribution of each predictor to model fit: Distance to intersection (χ2 = 4.204, p = 0.040), Type of CP (χ2 = 19.931, p < 0.001), and Distance to intersection: Type of CP (χ2 = 8.754, p = 0.013). Statistically significant results appear in bold. LCPs represent Locomotion CPs. FCPs correspond to both Frugivory and Gummivory CPs grouped together. OCPs represent all CPs other than LCPs and FCPs (i.e., Encounter CPs, Faunivory CPs, Fur-rubbing CPs, Vigilant CPs, Long-call CPs, Resting CPs, and Unknown CPs).Random effects: (0 + Distance to intersection|area): Variance = 0.5, SD = 0.708; (1|area): Variance = 0.001, SD = 0.001
3.4 Distance Traveled Between Fruit-Feeding Trees and CPs
We found that BLTs in larger areas traveled longer distances between both fruit-feeding trees and CPs (Figure 5A,B). However, BLTs from both the continuous and riparian forests spent similar numbers of consecutive scans traveling between the next fruit tree and the next CP. In the fragments, BLTs traveled for the highest number of consecutive scans before reaching the next CP (Figure 5C,D).
We found that the distances traveled between resources visited by BLTs varied across all study areas. In the continuous forest, BLTs traveled the largest distances between consecutive frugivory events (mean: 319.7 m ± SD 308.6 m), followed by the group in the medium fragment (mean: 122.6 m ± SD 139.8 m), small fragment (mean: 106.6 m ± SD 104.9 m), and the riparian forest (mean: 100.8 m ± SD 100.8 m) (Figure 5A). Even though the distances between sequential consumed fruiting trees in the riparian forest were shorter, we found that BLTs spent higher numbers of consecutive CPs traveling to the subsequent fruit feeding site (mean: 15.3 scans ± SD 12.5 scans). This pattern was similar to that observed in the continuous forest, where BLTs spent, on average, 15.0 scans ± SD 13.0 scans traveling between consecutive frugivory sites. On the other hand, we found BLTs to spend lower numbers of consecutive scans traveling between frugivory sites in the medium and small fragments, where frugivory bouts were, on average, separated by 7.9 scans ± SD 7.9 scans and 11.6 scans ± SD 10.2 scans, respectively (Figure 5B).
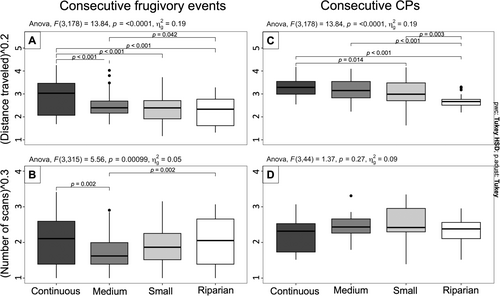
We found that BLTs traveled longer and shorter distances between consecutive CPs in the continuous forest (mean: 434.3 m± SD 261.3 m) and in the riparian forest (mean: 148.1 m ± SD 79.3 m), respectively. BLTs in the medium and small fragments traveled intermediary distances between consecutive CPs (mean: 400.3 m± SD 308.4 m; and mean: 332.2 m ± SD 251.2 m, respectively) (Figure 5C). Although not significatively different, we found that BLTs changed direction, on average, every 16.2 scans ± SD 10.1 scans in the continuous forest, while, in the riparian forest, the group changed direction with a mean of 19.6 scans ± SD 11.7 scans. On the other hand, BLTs spent the highest number of consecutive scans traveling without significatively changing direction in both the medium and small fragments (mean: 23.3 scans ± SD 14.7 scans; and mean: 26.2 scans ± SD 16.1 scans, respectively) (Figure 5D).
4 Discussion
By following different groups of wild BLTs living in contrasting forest fragments across the geographic range of this species, we found that feeding trees (i.e., fruit and gum feeding sites) are one of the main factors responsible for shaping their daily routes, regardless of the environmental context in which they live. We also found that BLTs seemed to use landmarks to redirect their routes, as shown by the proximity of LCPs to intersection points between daily trajectories, a result found for other platyrrhines (Abreu et al. 2021; Presotto et al. 2018). Particularly, in the continuous and riparian forests, which represented the highest and lowest values of used area, feeding tree richness and distances traveled between successive fruit feeding trees, BLTs used landmarks (here identified as route intersections) for route orientation while traveling between movement goals (or destinations). This pattern was different from the one observed in both medium and small fragments, with intermediate values of area used and distances traveled by BLTs to reach feeding trees, as well as the lowest values of fruit species richness.
As expected, we identified frugivory as the most important behavior shaping daily routes in the medium fragment, small fragment and riparian forest, while being the second most important in the continuous forest only after gummivory (Figure 3). Planning routes to reach important feeding sites, such as out of sight specific fruiting trees, has been reported for strepsirrhines (i.e., gray mouse lemurs (Microcebus murinus) (Joly and Zimmermann 2011)); catarrhines (i.e., northern pig-tailed macaques [Macaca leonina] [Albert et al. 2013]; white-handed gibbons [Hylobates lar] [Asensio et al. 2011]; chacma baboons [Papio ursinus] [Noser and Byrne 2014]; and western chimpanzees [Pan troglodytes verus] [Ban et al. 2016]); and platyrrhines (i.e., white-faced saki monkeys [Pithecia pithecia] [Cunningham and Janson 2007]; Geoffroy's spider monkeys [Ateles geoffroyi] [Boyer et al. 2006; Reyna-Hurtado et al. 2018]; Mexican spider monkeys [Ateles geoffroyi ssp. vellerosus] [Valero and Byrne 2007]; black-horned capuchin monkeys [Sapajus nigritus] [Janson 2016]; black-striped capuchins [Sapajus libidinosus] [Presotto et al. 2018]; black howler monkeys [Alouatta pigra] [Miguel de Guinea et al. 2021]; common marmosets [Callithrix jacchus] [Abreu et al. 2021]; and Weddell's saddleback tamarins [Saguinus fuscicollis weddelli] [Porter and Garber 2013]). The fact that the location of fruit feeding trees shaped BLT's daily trajectories corroborates our hypothesis and suggests that, regardless of the environmental context, BLTs possess intrinsic knowledge of the location of important out-of-sight feeding trees. Our results suggest that planning routes to reach specific out of sight fruit trees represents a crucial aspect of daily movement for a mostly frugivorous primate throughout the natural distribution of the species.
The high proportion of FCPs for shaping daily routes in all areas suggests that BLTs use directed paths between consecutive out-of-sight fruit and gum-feeding sites independently of the configuration of the environment. We also found FCPs to be located closer to route intersections than any other type of CP, suggesting that the locations of feeding trees might represent nodes on the route networks used by BLTs. Besides corresponding to important feeding sites for BLTs, nodes in route networks are also key locations constantly taken into consideration when deciding the path taken to reach the next traveling destination (Miguel de Guinea et al. 2021; Di Fiore and Suarez 2007; Noser and Byrne 2014). Moreover, in the case of both the medium and the small fragments, which are areas with intermediate distances between daily visited fruit-feeding trees, the low number of LCPs identified suggests that, in such areas, BLTs do not require additional types of landmarks for spatial orientation along their daily routes. Indeed, we found BLTs in these areas to present similar movement patterns along daily routes, traveling for longer periods and intermediate distances between frugivory sites without changing direction. Besides visual detection of primates being discussed to range between 35 m and 50 m (Miguel de Guinea et al. 2021; M. de Guinea et al. 2021; Hopkins 2011; Presotto et al. 2018), the visibility in forest remnants can vary significatively according to forest structure and seasonality. In fact, we have estimated visibility throughout the area used by the group in the small fragment to range between 5.5 and 24 m at breast height (mean = 15.1 m, SD = 5.0 m, N = 24) (Bufalo, unpublished data). It is important to consider that our sampling regime varied among groups, both in terms of intensity and distribution (the small fragment group was sampled only in one season) and that could have influenced our results. However, considering that all BLT groups oriented daily routes to travel between fruit feeding sites distant, on average, between 319.7 and 100.8 m from the previous frugivory location, our results indicate that BLTs could present spatial knowledge of the location of feeding sites and plan travel routes to efficiently navigate between out of sight feeding trees.
Although gummivory was recorded in less scans and represented fewer CPs than frugivory, it represented the most important type of CP in the continuous forest and the third most important in the riparian forest (Figure 3). As fruits, exudates are an important feeding resource for BLTs, corresponding to up to 22% of their diet (Mamede-Costa 1997; Martins 2003; Passos 1999; Silva 2022). Gum is known to be an important source of sugars, proteins, minerals, and water (Dewi et al. 2022; Ganzhorn et al. 2023; Garber 1984), and has been pointed out to have some beneficial pharmaceutical properties for primates (Ganzhorn et al. 2023). Also, other primate species have already been pointed out to plan routes to reach important gum feeding trees, as in the case of gray mouse lemurs (Microcebus murinus) (Joly and Zimmermann 2011) and Javan slow loris (Nycticebus javanicus) (Poindexter et al. 2023). Considering the importance of such resource on BLTs’ diet, it is not surprising that BLTs are also directing routes to reach gum feeding trees. In fact, our results indicate that 11% of BLTs' gummivory events in the continuous forest were preceded by directed routes. As a comparison, 7% of the frugivory events in this area were a consequence of directed routes. Although less frequent, we also found a high importance of exudates on route planning by BLTs in the riparian forest, where BLTs directed routes to 3% of their gummivory events. However, we did not find such great importance of gummivory for shaping daily routes in the two fragments (medium and small forest). The contrasting differences in gummivory observed between continuous and riparian forests and the fragments might be a consequence of a reduced richness of feeding trees in the fragments and/or due to seasonal differences in sampling. Further studies would be important to confirm these trends.
We identified LCPs to be mostly distributed closer to route intersections and to marginally differ from FCPs. Also, LCPs were the second most important CPs in both the continuous and the riparian forests. This fact corroborates our hypothesis, indicating that LCPs are more concentrated near points of spatial orientation along the trajectories. The fact that CPs associated with locomotion were more frequent in the continuous and the riparian forests suggests that BLTs rely more on spatial features of the landscape, possibly landmarks, for route orientation in these two areas compared to the fragments, but not only to reach fruit feeding trees, as in the case of riparian forest. This slightly contrasting pattern between the medium and the small fragments and the continuous and riparian forests might be explained by characteristics of the areas known to influence the movement of primates, such as the structure (McLean et al. 2016), size, and shape of the forest (Arroyo-Rodríguez and Mandujano 2006), the size of the used area, the probability of encounter with other conspecific groups (Sobral, Fuzessy, and de Oliveira 2023), and the richness and distribution of fruit resources (Reyna-Hurtado et al. 2018).
In the continuous forest, where BLTs used the largest area and traveled the largest distances daily to reach sparsely distributed resources, efficiently navigating through the landscape can be cognitively demanding (Milton 1981). In fact, BLTs in this area displayed the highest CP Ratios associated with reaching feeding trees, such as frugivory and gummivory CPs, among all study areas (Figure 3). These findings indicate that BLTs in the continuous forest planned and used directed routes to reach out-of-sight feeding sites proportionally more compared to the other forest areas. On the other hand, we also found BLTs in the continuous forest to rely on the use of landmarks for route orientation more often than in any other study area. Landmarks can represent relatively stable structures in the landscape across time, serving as reliable orienting features for BLTs when traveling long distances between objectives (Dolins and Mitchell 2010). In a large area such as the continuous forest, BLTs might be using landmarks for reorientation along travel routes between successive out-of-sight feeding trees (Bertolani 2013; Erhart and Overdorff 2008). BLTs could also benefit from the use of landmarks as they allow for the group to take detours to check for the availability of resources in proximity and monitor the borders of the territory for the presence of conspecific groups (Cunningham and Janson 2007; Noser and Byrne 2007). Therefore, relying on landmarks for daily routes' orientation could be beneficial for conserving energy and optimizing travel efficiency when traveling between different types of out of sight destinations, particularly in a large continuous forest (Porter and Garber 2013).
We obtained a similar result for the riparian forest, with BLTs exhibiting a high frequency of LCPs along daily routes. Contrastingly, BLTs in this study site presented the smallest area used among the four areas included in this study. Also, we found BLTs in the riparian forest to travel significantly shorter distances between fruit-feeding sites than in the continuous forest. While the relatively small area used and the short distances traveled between feeding resources could suggest that BLTs would be able to use routes directed to fruit feeding sites, the group in the riparian forest presented a similar proportion of LCPs as observed for BLTs' in the continuous forest, while also often using landmarks for route orientation between significatively shorter distances. Accounting for other possible explanations for the high frequency of LCPs in the riparian forest and considering that BLTs in this area are highly constrained into a narrow riparian forest remnant, we tested for a possible association between LCPs and the distance to forest limits with the matrix. Also, considering the importance of territory defense and resource monitoring in the daily activities of callitrichids (Garber and Porter 2014; Peres 1989), we tested if LCPs were concentrated closer to the borders of both area used and core area. We found that CPs in the riparian forest were not determined by physical or social limits of the environment (Supporting Information S1: Table S9). Conversely, other types of forest vegetation structure and canopy discontinuity along daily routes might also be influencing the decisions of where to turn along trajectories and should be investigated by future research (Harel et al. 2022; McLean et al. 2016).
Finally, we found the group in the small fragment to present contrastingly lower frequencies of resting in both scans and CPs when comparing all four areas. Resting is considered to be an important aspect of the life of wild primates, allowing for social interactions to be reinforced, while also providing room for important physiological processes (Herbers 1981; Korstjens, Lehmann, and Dunbar 2010). Nevertheless, resting can correspond to an important portion of the activity period of wild animals, therefore representing a cost in terms of resource acquisition (Herbers 1981). Indeed, it has been pointed out that resting-related activities (i.e., resting or performing social activities) are determined, among other factors, by the richness and availability of resources (Roberts and Dunbar 1991), with primates resting more in areas with higher richness and availability of feeding resources (Dunn, Cristóbal-Azkarate, and Veà 2009; Hill 1999; Irwin 2008). Yet, we found BLTs in the small fragment to consume the lowest richness of fruits and to present a higher frequency of faunivory than in any other area. While further investigations are necessary to draw conclusions, our results corroborate previous findings of primates compensating the nutrient intake from the available fruit species by relying more on alternative feeding items (Bicca-Marques, Chaves, and Hass 2020; de Luna et al. 2017; Irwin 2008; Onderdonk and Chapman 2000; Tutin 1999; Umapathy and Kumar 2000). Our results suggest that BLTs in the small fragment might need to plan daily routes focused on a higher investment into searching for animal prey and, consequently, leaving less time available for resting in an environment with low fruit richness. Reduced resting has been shown to lead to cognitive impairment in humans (Alhola and Polo-Kantola 2007; Killgore 2010), rhesus macaques (Macaca mullata) (Promsote et al. 2023), rodents (Patti et al. 2010; Rossi et al. 2014), birds (Johnsson et al. 2022), and fish (Pinheiro-da-Silva, Tran, and Luchiari 2018), and could also affect BLTs' ability to memorize, among other important ecological aspects of the environment, the phenological state of important feeding trees. Further investigations are necessary to better understand the long-term consequences of reduced resting on the cognition of wild BLTs in forest fragments.
In this study, we investigated BLT groups in four distinct environmental contexts across the geographical distribution of the species. Our results indicate that BLTs may recognize landmarks (route intersections) leading to important feeding sites, and use them consistently for route planning. We also found that BLTs orient their routes in a relatively similar way across forests of different sizes. However, there are noticeable differences in route planning depending on the environmental context. In larger areas (i.e., continuous forest), where resources are more sparsely distributed, the use of landmarks seems to become an important factor in shaping routes. On the other hand, in areas where movement is restricted due to habitat configuration (i.e., narrow patches of forest, such as in the riparian forest studied) and resources are closer together, BLTs appear to have less need to plan routes to reach fruit-feeding trees. Under these conditions, other structural aspects of the environment, such as canopy discontinuity, which can be a barrier to movement, may explain the higher frequency of LCPs (Davies et al. 2017; McLean et al. 2016). Our results provide the first evidence for the repeated use of landmarks for route guidance based on a network system in BLTs. We conclude that, depending on the context, BLTs plan daily routes differently to achieve travel goals and increase route efficiency to reach out-of-sight feeding trees, potentially maximizing a nutrient-balanced diet.
Author Contributions
Felipe Bufalo: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (lead), Investigation (lead), methodology (lead), project administration (lead), visualization (lead), writing–original draft (lead); Writing–review and editing (lead). Olivier Kaisin: investigation (equal), methodology (equal), writing–review and editing (equal). Anne-Sophie Almeida e Silva: investigation (equal), methodology (equal). Rodrigo Gonçalves Amaral: investigation (equal), methodology (equal). Yness Messaoudi: investigation (equal). Mirela Alcolea: investigation (equal). Eduardo M. Zanette: methodology (equal), Writing–review and editing (equal). Gabriel Pavan Sabino: investigation (equal), writing–review and editing (equal). Luca Börger: methodology (equal), writing–review and editing (supporting). Laurence Culot: conceptualization (lead), funding acquisition (lead), investigation (lead), methodology (lead), project administration (lead), resources (equal), supervision (lead), writing–review and editing (lead).
Acknowledgments
We thank Neli Fidencio Rodrigues and Ronald Teles Rodrigues, as well as other family members, for all support during data collection in Guareí. We also thank the valuable contribution provided by the Editor Dr. Pedro Dias, and two anonymous reviewers, for the improvement of this manuscript. This work was supported by the São Paulo Research Foundation (FAPESP—Young Investigator Grant #2014/14739-0 and #2021/06668-0 to Laurence Culot). Felipe Bufalo received a fellowship from the São Paulo Research Foundation (FAPESP #2023/01760-0), from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES #88881.846203/2023-01) and from the Brazilian National Council for Scientific and Technological Development (CNPq: #133172/2018-0 and #443489/2020-3). Olivier Kaisin received funding from the National Fund for Scientific Research (FRS-FNRS, Belgium). Anne-Sophie Almeida e Silva received a fellowship from CNPq (#141813/2017-2) and a Small Grant from the Rufford Foundation (#29108-1). Rodrigo Gonçalves Amaral received a fellowship from FAPESP (#2019/11102–5 and #2023/13054-3) and from (CAPES). Eduardo M. Zanette received fellowships from CNPq (#130909/2020-3) and FAPESP (#2020/11129-8 and #2021/10284-2). Laurence Culot Received a Research Productivity Fellowship from CNPq (#314964/2021-5). We thank Instituto de Pesquisas Ecológicas (IPE) for the logistics and field support.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




