Hepatobiliary Platynosomosis in Black-Tufted Marmosets (Callithrix penicillata): A Lethal Threat for Wildlife and Captive Populations
ABSTRACT
Helminthic infections, particularly those caused by trematodes, pose a significant health risk to both animals and humans. This study investigates hepatobiliary platynosomosis (HP) caused by Platynosomum illiciens in black-tufted marmosets (Callithrix penicillata) in Central Brazil. Data were retrospectively collected from autopsy records at the Laboratory of Veterinary Pathology and Forensics, University of Brasília, from January 2006 to July 2021. Epidemiological, clinical, and pathological information was analyzed, including comparisons between free-ranging and captive marmosets. A total of 1596 nonhuman primate (NHP) death records were examined, with black-tufted marmosets accounting for 75.6% (1206/1595) of autopsies. HP was identified in 10.8% (131/1206) of these cases. Captive marmosets showed a higher prevalence of HP (49.6%) than free-ranging ones (6.5%). This study revealed a significant seasonal trend, with higher HP prevalence observed during the wet season until the onset of the dry season. Pathological examinations revealed severe liver and bile duct damage in fatal HP cases, including fibrosis, bile duct thickening, and the presence of flukes. Captive marmosets exhibited pronounced clinical signs, such as weight loss and apathy. Morphological and molecular analysis of sampled flukes confirmed P. illiciens infecting the marmosets. These findings underscore the substantial impact of HP on marmoset populations, particularly in captive settings. The high lethality (58% overall; 81.6% in captivity) highlights the need for improved management and preventive measures in captive environments. This study contributes to understanding HP epidemiology, clinical manifestations, and pathological outcomes, underscoring the disease's significance for the health of both free-ranging and captive NHP populations. Our findings also support the need to develop targeted strategies to mitigate the impact of HP on primate species populations.
Summary
-
Hepatobiliary platynosomosis (HP) affects free-ranging and captive marmosets and should be considered a major disease for free-ranging and captive animals.
-
Epidemiologic and clinic-pathological findings of HP in nonhuman primates are scarce worldwide.
-
HP prevalence ranged from 5.1% to 31.4% in free-ranging marmosets, with severe weight loss and hepatic damage in fatal cases.
Abbreviations
-
- H&E
-
- hematoxylin and eosin
-
- HP
-
- hepatobiliary platynosomosis
-
- LVPF
-
- Laboratory of Veterinary Pathology and Forensics
-
- NHP
-
- nonhuman primates
-
- PC-UnB
-
- Primatology Center, UnB
-
- UnB
-
- University of Brasilia
1 Introduction
Helminthic infections, particularly those caused by trematodes, represent a significant yet often overlooked threat to both animal and human health globally (de Sousa et al. 2021; Tidman et al. 2023; Manga-González, Ferreras, and Kafle 2024). Platynosomum illiciens is a digenetic trematode that parasitizes the hepatobiliary tract of mammals and birds in tropical and subtropical regions worldwide (Silva, Silva, and Pereira 2012a; Pinto, Mati, and de Melo 2014; Pinto, Melo, and Mati 2022). As a trematode with low host specificity, P. illiciens infections have increased over time due to the anthropogenic spread of potential hosts, including invasive species, facilitating the parasite's expansion across different environments (Pinto, Melo, and Mati 2022).
Domestic cats are recognized as primary definitive hosts in the veterinary practice, but this parasite has also been identified in both platyrrhine and catarrhine primates, indicating a broader host range and potential health implications for wildlife (Warren et al. 1998; Sousa et al. 2008; Mati, Pinto, and Melo 2015; Mattioli et al. 2016; Ramos et al. 2016; Pereira et al. 2021). In captive environments, hepatobiliary platynosomosis (HP) is often considered asymptomatic (Silva, Silva, and Pereira 2012a; Pereira et al. 2021; Pinto, Melo, and Mati 2022) however, it can lead to significant pathological changes, affecting the gallbladder, bile ducts, and hepatic parenchyma (Sousa et al. 2008; Pereira et al. 2021). Fatal outcomes have rarely been reported as isolated HP cases in free-ranging nonhuman primates (NHPs) (Assis et al. 2021).
The current understanding of the epidemiology, clinical presentation, and pathological impacts of HP in free-ranging NHPs is limited. This gap in knowledge underscores the need for comprehensive studies to elucidate the disease's prevalence, impact on population health, and mechanisms of pathogenesis. This study aims to address these gaps by investigating the epidemiological, clinical, pathological, and etiological characteristics of HP in both free-ranging and captive black-tufted marmosets (Callithrix penicillata) in Central Brazil. Understanding these aspects is crucial for developing effective conservation strategies and improving the management of affected populations.
2 Material and Methods
A retrospective survey of HP cases in black-tufted marmosets (C. penicillata) was conducted utilizing data from the archives of the Laboratory of Veterinary Pathology and Forensics (LVPF), University of Brasilia (UnB), Federal District, Brazil, from January 2006 to July 2021. Epidemiological, clinical, and pathological information, including sex, age group (juvenile or adult), origin (free-ranging or captive), year, month, and geolocation, were retrieved from clinical (for captive marmosets), autopsy, and epidemiological records. Marmosets elected for the study exhibited HP, irrespective of the cause of death. When data records were incomplete, available information was partially utilized or excluded from frequency and statistical analyses.
LVPF-UnB serves as an Official Regional Laboratory under the Ministry of Health of Brazil for the Diagnosis of Yellow Fever and other epidemics in NHPs. It receives free-ranging NHPs found dead, collected by State Health Surveillance Services (Federal District and the States of Goiás, Mato Grosso, and Tocantins) as part of the Brazilian National Program for Yellow Fever Control. Captive marmosets included in this study originated from the Primatology Center, UnB (PC-UnB), situated within an environmental reserve area. Animals found dead within the animal facilities underwent routine autopsy examinations during the study period, accompanied by history and clinical records. Evaluation of PC-UnB facilities was conducted to assess conditions and management practices that could contribute to the spread of infection among captive animals.
All cases of HP were categorized as either fatal or non-fatal. Fatal cases encompassed marmosets exhibiting moderate to severe gross and microscopic hepatic lesions attributed to P. illiciens infection, cachexia (evidenced by marked loss of muscle mass and fat tissues), and without other concurrent diseases (both free-ranging and captive animals), accompanied by a history of chronic weight loss in captive animals. non-fatal cases of HP showed mild to moderate hepatic lesions and moderate to good body condition scores, and they died due to causes other than HP, such as traumatic injuries, electrocutions, and predation, among others. In captive animals with fatal HP and complete records from the animal facilities, monthly body weights were assessed to evaluate the impact of infection on marmosets' weight, as well as to track the progression from the onset of clinical signs to death.
During autopsies, tissue samples were collected, fixed in 10% buffered formalin, routinely processed, embedded in paraffin, and stained with hematoxylin and eosin (H&E). Liver samples were also stained with Masson's trichrome and reticulin stain. All gross liver lesions were documented. Microscopic changes were semiquantitatively assessed as absent (–), mild (+), moderate (++), and severe (+++). All NHPs tested negative for the Yellow Fever virus via immunohistochemical assay conducted on liver samples in this study.
Flukes identified within the gallbladder and hepatic bile ducts were collected for morphological and molecular characterization in HP cases spanning all study years. Flukes collected throughout the study period were aggregated for convenience into eight pools for free-ranging marmosets and three pools for captive animals. For morphometric analysis and parasite identification, trematodes were preserved in an alcohol-formalin-acetic acid solution (comprising ethanol 95%, formaldehyde 3%, and glacial acetic acid 2%) and clarified in 80% glacial acetic acid. Subsequently, parasite samples were subjected to brightfield microscopy and morphometry using ImagePro Plus 4.0 software.
Pools of trematodes were preserved by freezing at −20°C in a sterile 0.9% saline solution for subsequent molecular identification and analysis. These samples were organized into 12 pools for free-ranging animals and three pools for captive animals, grouped by convenience and arranged in chronological order according to the year of collection. Trematode DNA was extracted from the pools using the Illustra Tissue & Cells GenomicPrep Mini Spin Kit (GE Healthcare Life Sciences do Brasil Ltda, Brazil).
Approximately 396 bp long fragments of the mitochondrial cytochrome c oxidase 1 gene (cox1) were amplified with the previously described primers JB3 and JB5 (Pinto et al. 2018). Conventional PCR assays were carried out in 25 µL reaction volume containing 10× PCR buffer, 1.0 mM MgCl2, 0.25 mM deoxynucleotide triphosphate (dNTPs) mixture, 1.25 U Taq DNA Polymerase (Invitrogen®), 1.0 µM of each primer, and 1.0 µL of DNA—used as a template. PCR products were separated by electrophoresis in 2% agarose gels, stained with ethidium bromide, and examined under ultraviolet light. The PCR products were purified from gel using PureLink™ Quick Gel Extraction & PCR Purification Combo Kit (Invitrogen by Thermo Fisher Scientific, Lithuania) and submitted for Sanger sequencing using the forward and reverse primers.
The chromatogram and quality scores of the sequences were assessed, and manual trimming was performed. The identity and E-values were evaluated with the BLASTn tool. A phylogenetic tree was constructed based on the partial cox1 nucleotide sequence to assess the taxonomic position of these trematodes. We retrieved 22 representative isolates of the family Dicrocoeliidae from GenBank. The alignments were carried out using the ClustalW algorithm. A maximum-likelihood tree was constructed in RAxML v8.2 software (Kozlov et al. 2019) and rooted with Fasciola hepatica (MW86731). The best substitution model was used according to the jModelTest v2.1.10 tool. Bootstrap analysis was performed with 1000 replicates, and the convergence was assessed. The sequences of this study were deposited in the GenBank database under the accession numbers PP837838, PP837839, PP837840, P837841, PP837842, PP837843, PP837844, PP837845, PP837846, PP837847, PP837848, PP837849, PP837850, PP837851 and PP837852.
The spatial distribution of HP cases was analyzed based on the geolocation data of free-ranging marmoset deaths using the QGIS 3.16 software. Cases were categorized according to the location of deaths into urban, peri-urban, natural areas, and captivity. Prevalence and lethality rates were calculated based on the number of animals with HP among the total deaths of marmosets during the study period.
Epidemiological data frequencies and pathological liver changes were compared between fatal and non-fatal HP cases and between free-ranging and captive animals using the Fisher's exact test, conducted using the GraphPad Prism® 8.01 software. Liver injury intensities among fatal and non-fatal cases and between free-ranging and captive animals were compared using the Wilcoxon Rank Sum Test, a non-parametric test performed with the R Core Team (2019) software, along with the Dunn's test packages (https://CRAN.R-project.org/package=dunn.test) and RVAideMemoire (https://CRAN.R-project.org/package=RVAideMemoire).
3 Results
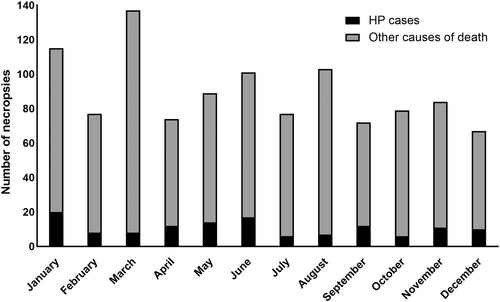
During the study period (2006–2021), a total of 1596 NHP death records were assessed. Black-tufted marmosets constituted 75.6% (1206/1595) of all autopsies, with 10.8% (131/1206) showing HP. Among the animals with HP, 54.2% (71/131) were free-ranging marmosets, while 45.8% (60/131) were in captivity. Details regarding the origin, sex, and age group of the affected marmosets are provided in Table 1. In this study, the temporal analysis did not reveal any differences in the prevalences of HP between wet (from October to April) and dry (from May to September) seasons (p > 0.05) in free-ranging and captive animals, fatal and non-fatal cases, and in the totality of HP cases in marmosets (Figure 1).
| Fatal n=(%) | Non-fatal n = (%) | Total n = (%) | ||
|---|---|---|---|---|
| Origin | ||||
| Captive | 49 (39.4%) | Captive | 11 (8.4%) | 60 (45.8%) |
| Free-ranging | 27 (20.6%) | Free-ranging | 44 (33.6%) | 71 (54.2%) |
| Sex | ||||
| Female | 36 (27.5%) | Female | 30 (22.9%) | 66 (50.4%) |
| Male | 32 (24.4%) | Male | 21 (16.0%) | 53 (40.4%) |
| Not recorded | 8 (6.1%) | Not recorded | 4 (3.1%) | 12 (9.2%) |
| Total | ||||
| Age group | ||||
| Adult | 57 (43.5%) | Adult | 30 (22.9%) | 87 (66.4%) |
| Juvenile | 1 (0.8%) | Juvenile | 8 (6.1%) | 9 (6.9%) |
| Not recorded | 18 (13.7%) | Not recorded | 17 (13.0%) | 35 (26.7%) |
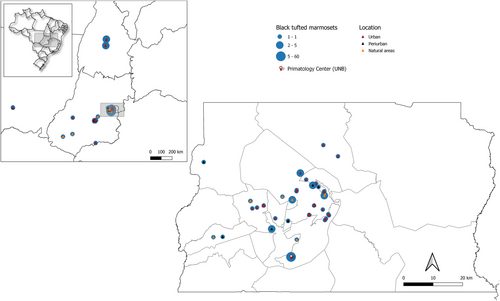
The prevalence of HP was 6.5% (71/1085) in free-ranging marmosets and 49.6% (60/121) in captive animals (p < 0.05). Considering only free-ranging marmosets in Central Brazil, HP prevalence was 5.5% (48/872) in the Federal District (DF), 5.1% (10/196) in Goiás (GO), 12.5% (2/16) in Mato Grosso (MT), and 31.4% (11/35) in the Tocantins (TO) States. Among HP cases with geolocation data (n = 54), 63.0% (34/54) occurred in urban areas, 18.5% (10/54) in peri-urban regions, and 18.5% (10/54) in natural areas. The geographical distribution of HP cases in Central Brazil is shown in Figure 2.
The affected marmosets exhibited a lethality of 58.0% (76/131), with 38.0% (27/71) observed in free-ranging and 81.6% (49/60) in captive animals. An epidemiological investigation conducted at the UnB Primatology Center revealed animal enclosures with metal screens that could facilitate the access of intermediate hosts. The ground in most enclosures retained moisture and minimal organic matter, harboring numerous isopods.
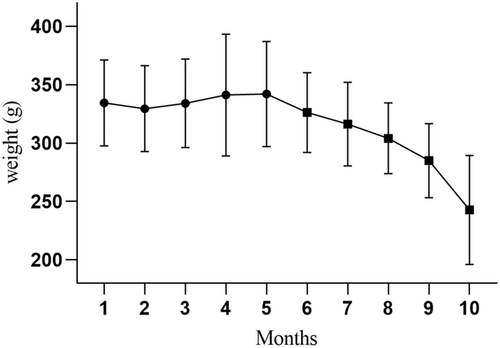
Clinical signs observed in captive animals with HP included progressive weight loss and apathy in 81.1% (43/53) of cases. Seven animals (11.5%) had no clinical information in the records. Weight evaluation of 12 captive animals with fatal HP over 10 months evidenced a significant body weight loss 5 months before death (p < 0.001) (Figure 3). The first clinical signs and body weight loss were noted in these captive animals between the fifth and sixth month before death (p < 0.05). The average body weight loss was 27.8% ± 11.0% in affected captive marmosets from the onset of clinical signs to death.
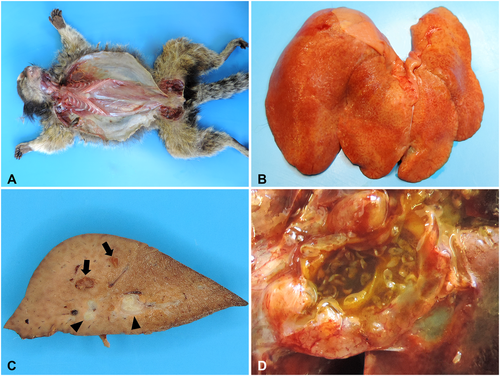
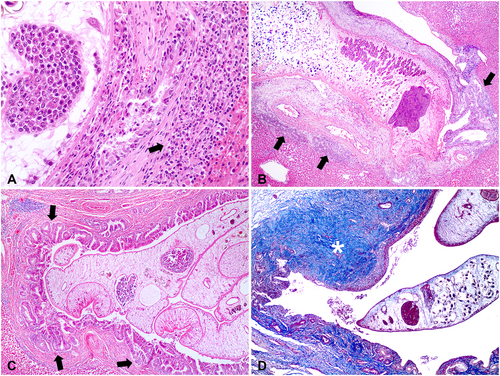
Grossly, cachexia was the most prominent change in fatal HP cases (Figure 4A). Enhanced lobular pattern (Figure 4B) was observed in 90.9% (50/55) of cases, along with fibrosis (Figure 4C) in 85.5% (47/55), thickening of the bile ducts (Figure 4C) in 72.8% (40/55), ductal ectasia in 72.8% (40/55) (Figure 4C), overdistended gallbladder by bile in 70.9% (39/55), and visualization of flukes within the gallbladder and bile ducts (Figure 4D) in 34.6% (19/55) of cases, representing the primary platynosomosis-associated liver lesions identified in infected marmosets.
Hepatic histopathological findings observed in fatal (n = 76) and non-fatal (n = 55) HP cases and free-ranging (n = 71) and captive (n = 60) black-tufted marmosets are shown in Figure 5A,B. Differences in frequencies were detected among some of the lesions (p < 0.05). Periportal lymphoplasmacytic inflammatory infiltrates (Figure 6A), ectasia and ductal proliferation (Figure 6B), bile duct epithelium hyperplasia (Figure 6C), and fibrosis (Figure 6D) were the most notable histological lesions observed in the livers of infected marmosets. Additionally, 31.29% (41/131) of animals exhibited biliary epithelial necrosis (Figure 7A), predominantly at the site of fluke attachment (Figure 7B) in fatal cases.
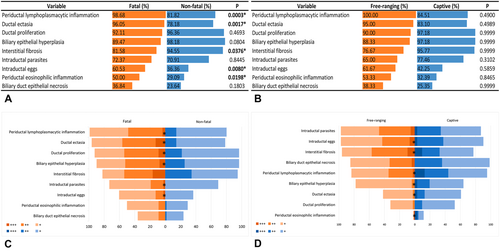
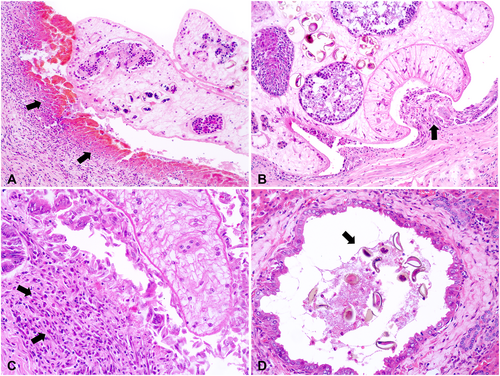
Biliary duct proliferation, ectasia, and epithelial hyperplasia, along with lymphoplasmacytic and eosinophilic periductal infiltrate (Figure 7C) and the presence of fluke eggs within biliary ducts (Figure 7D), were more pronounced in animals with fatal outcomes compared to non-fatal cases (p < 0.05) (Figure 5C). Hepatic fibrosis and ductal epithelial hyperplasia were more severe in free-ranging marmosets than in captive animals (p < 0.05). Ductal ectasia, fluke eggs within biliary ducts, and periductal mononuclear and eosinophilic inflammatory infiltrates were more intense in captives than in free-ranging animals (p < 0.05) (Figure 5D). Biliary epithelial hyperplasia, lymphoplasmacytic cholecystitis, and free or attached flukes were the most significant pathological findings in the gallbladder, but there were no differences in the frequencies and intensities of these changes between fatal and non-fatal cases (p > 0.05), and free-living and captive animals (p > 0.05).
Morphological analysis of flukes (Figure 8) demonstrated a total length (mm) of 4.89 ± 1.56 and a width of 1.71 ± 0.39. The oral sucker had 0.39 ± 0.07 length x 0.40 ± 0.06 width, the esophagus 0.63 ± 0.26, the ventral sucker 0.46 ± 0.06 length x 0.47 ± 0.05 width, right vitelline gland 1.02 ± 0.39 length x 0.35 ± 0.10 width, and left vitelline gland 1.01 ± 0.43 length x 0.34 ± 0.12 mm width. The right testis showed 0.62 ± 0.24 length x 0.43 ± 0.14 width, left testis 0.61 ± 0.23 length x 0.43 ± 0.13 width, and ovary 0.27 ± 0.08 length x 0.32 ± 0.12 width.

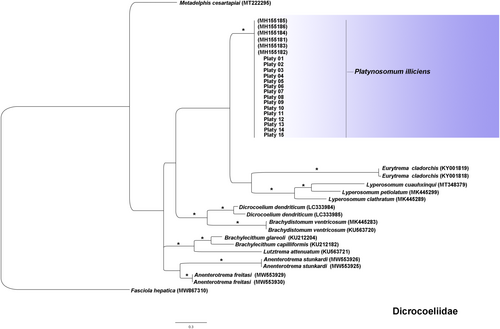
Molecular identification showed no double peak in the chromatogram, ruling out the need for molecular cloning. The 15 sequences identified in this study showed high nucleotide identity (100%) among them and with other P. illiciens isolates (99.7%–100%) in the multiple alignments (Figure 9). Low identity was observed with the closest species in the Dicrocoeliidae family, Lyperosomum spp. (80.7%–82.8%), Dicrocoelium dendriticum (80.2%–81%) and Eurytrema cladorchis (79%). The isolates of the present study are grouped in a monophyletic cluster supported by a high bootstrap compared to other P. illiciens isolates.
4 Discussion
The investigation of diseases affecting wildlife populations, particularly free-ranging animals, is of great relevance and can sometimes reveal emerging or under-recognized illnesses impacting these animals. One such example is the scenario demonstrated by this study regarding HP in marmosets in Central Brazil. In our research, infections by P. illiciens showed a frequency exceeding 10%, with notably higher prevalence among captive animals compared to free-ranging NHPs. Additionally, over 80% of free-ranging animals with HP were found dead in urban and peri-urban areas. P. illiciens parasitism in free-ranging marmosets ranged from 5.1% to 31.4% across three states and the Federal District.
A survey of free-ranging NHPs from Rio de Janeiro reported a prevalence of 8.9% (Oliveira et al. 2023). Another parasitological study on free-ranging callitrichids from urban areas and forest fragments observed a prevalence of 5.4% of infected animals by Platynosomum sp. (Pereira et al. 2020). In most studies, only isolated cases of HP have been observed in free-ranging Callithrix sp. in urban and natural areas in the Southeast region of Brazil (Melo 2004; Díaz-Delgado et al. 2018; Oliveira et al. 2019; Assis et al. 2021).
Regarding the prevalence of HP in free-ranging NHPs, most available studies are limited and have primarily been conducted in regions and under conditions different from those in this study carried out in the Cerrado Biome (Brazilian Savannah), making direct comparison difficult. This study showed variations in HP prevalence across different states, which may be attributed to smaller sample sizes from specific locations (e.g., Tocantins State), potentially resulting in higher detected infection rates. Additionally, these differences could be influenced by undetermined local factors. Nevertheless, the prevalence of HP in Central Brazil was particularly notable, considering free-ranging marmosets and its possible impact on wild populations.
The high prevalence of HP in captive NHPs is likely due to challenges in controlling the infection of NHPs within the Primatology Center, UnB animal facilities. In captive callitrichids, the prevalence of HP has not yet been determined, but its occurrence has been described in C. penicillata, C. jacchus, and C. geoffroyi in the Federal District and the states of Minas Gerais, Pará, Rio de Janeiro, Rio Grande do Norte, São Paulo, and Espírito Santo (Sousa et al. 2008; Silva, Silva, and Pereira 2012a; Mati, Pinto, and Melo 2015; Mattioli et al. 2016; Díaz-Delgado et al. 2018; Pinto et al. 2017; Pinto et al. 2018; Assis et al. 2021; Pereira et al. 2020; Pereira et al. 2021).
HP has also been described in other captive platyrrhine primates such as Black bearded saki (Chiropotes satanas), Titi monkey (Callicebus moloch), Goeldi's marmoset (Callimico goeldii), and Pygmy marmoset (Cebuella pygmaea) in the state of Pará, Brazil (Silva, Silva, and Pereira 2012a; Pereira et al. 2021). In catarrhine primates, Platynosomum sp. infections were observed in three orangutans (Pongo pygmaeus) in Indonesia (Warren et al. 1998) and in a female Macaca fascicularis kept as a pet in Malaysia (Shanta 1970). Compared with other studies on HP affecting various captive primate species, it was not possible to determine specific differences in management or environmental conditions that could explain the observed prevalence in this study.
Concerning the absence of seasonality in HP cases observed in this study, the broad distribution and high prevalence of P. illiciens infections suggest the existence of favorable environmental conditions for the parasite's life cycle. These include an abundance of intermediate and paratenic hosts, as well as suitable climatic and ecological factors in both urban and natural habitats. These factors may account for the similar monthly distribution of HP cases across all conditions evaluated. Currently, studies addressing the seasonality of HP in NHPs remain limited.
The infection of animals in our study likely occurred through the ingestion of mollusks, isopods, or lizards, which are considered intermediate and paratenic hosts for Platynosomum spp. (Rosenberger 1992; Pinto, Mati, and de Melo 2014; Pinto, Mati, and Melo 2015). Small vertebrates, invertebrates, and insects, including lizards and certain mollusk species, serve as paratenic and intermediate hosts for Platynosomum spp. (Pinto, Mati, and de Melo 2014) and are considered natural prey for free-ranging Callithrix spp. (Passamani and Rylands 2000). Some of these intermediate hosts (isopods) were observed in the facilities of the evaluated primates kept in captivity.
The high frequency of HP observed in captive marmosets in our study demonstrates the risks that the absence of adequate prevention and control measures in the facilities, access to intermediate hosts, and animal overcrowding can promote high infection rates and lethality within the populations. To prevent the high prevalence of HP observed in marmosets at the Primatology Center, UnB, identifying and treating infected animals, as well as implementing changes to the facilities to prevent the entry and presence of intermediate/paratenic hosts, are measures that could potentially aid in controlling the disease.
The lethality of HP in the animals assessed in the study approached 60%. Animals in captivity infected with P. illiciens showed high lethality (above 80%), whereas in free-ranging NHPs, despite notable values (38.0%), the lethality rate was less than half of that observed in captive animals. Fatal cases of the disease were rarely reported in free-ranging NHPs (Oliveira et al. 2019) and more frequently in animals kept in captivity (Sousa et al. 2008; Mattioli et al. 2016; Pereira et al. 2021). In contrast to what was observed in our study, HP has been described as a subtle gross finding in autopsies not directly related to the death of marmosets (Díaz-Delgado et al. 2018; Assis et al. 2021).
No predisposition by sex was observed among fatal and non-fatal cases of HP, and most animals were adults in both outcomes. These findings were similar to those observed in naturally infected captive animals by P. illiciens (Mattioli et al. 2016). The high lethality detected in our study for both free-ranging and captive marmosets demonstrates the risk that HP poses to NHP populations. Under the conditions of this study, the factors influencing the transmission and lethality of HP in both captive and free-ranging NHP populations within anthropogenic environments could not be determined and remain to be further investigated.
Over 80% of infected captive animals displayed clinical signs such as weight loss and apathy. In addition to lethargy, reduced adipose tissue, and weight loss, captive C. penicillata and C. jacchus infected with P. illiciens also exhibited symptoms like anorexia, dehydration, alopecia, muscle wasting, abdominal sensitivity, diarrhea, and jaundice (Sousa et al. 2008; Mati, Pinto, and de Melo 2021). The pronounced weight loss and clinical progression observed over 5 months in captive marmosets with HP in this study were previously observed in six C. jacchus with the disease over a period ranging from 2 to 11 months (Sousa et al. 2008). Similar clinical signs have been reported in other captive and free-ranging South American NHPs with HP, including C. geoffroyi (Oliveira et al. 2019), Callithrix sp. (Díaz-Delgado et al. 2018), captive black-tufted marmosets (Sousa et al. 2008; Pereira et al. 2021), C. satanas and C. pygmaea (Silva, Silva, and Pereira 2012a; Silva et al. 2012b), as well as P. pygmaeus in Indonesia (Warren et al. 1998).
The primary clinical signs observed in both free-ranging and captive marmosets with HP are generally nonspecific and associated with other conditions resulting in chronic weight loss. Wasting marmoset syndrome (WMS) is an important multifactorial disease characterized by chronic weight loss, which affects callitrichid colonies globally (Sousa et al. 2008; Niimi and Takahashi 2021) and should be considered the main differential diagnosis for HP. However, despite the overlap in clinical presentation, while diarrhea and intestinal inflammation are common in WMS, they are not observed in HP cases (Sousa et al. 2008; Niimi and Takahashi 2021), as noted in this study. Furthermore, the clinical signs and harmful effects of P. illiciens infection underscore its often fatal impact on severely affected animals.
Among the gross changes observed in animals with HP, the most relevant findings included the enhancement of the hepatic lobular pattern, fibrosis, ectasia, thickening of the biliary ducts, and distended gallbladder. These gross findings were also recorded in infected C. penicillata (Silva, Silva, and Pereira 2012a; Pereira et al. 2021), C. jacchus (Sousa et al. 2008), C. geoffroyi (Pereira et al. 2021; Oliveira et al. 2019), and other NHPs such as C. satanas (Pereira et al. 2021). Notably, no significant gross liver alterations beyond the visualization of parasites during autopsy were found in many non-fatal cases in our study. Captive Callithrix sp. (Assis et al. 2021), C. moloch, C. goeldii, C. satanas (Pereira et al. 2021), and P. pygmaeus (Warren et al. 1998) with HP also showed no gross hepatic changes. Determining gross hepatic alterations associated with HP in marmosets requires further investigation, particularly regarding their relationship to clinical progression, lesion severity, and outcomes.
Microscopically, the most frequent hepatic histopathological findings in all infected animals included periductal mononuclear and eosinophilic inflammatory infiltrate, ectasia, epithelial hyperplasia, biliary duct proliferation, intraductal fluke eggs, and varying degrees of fibrosis. These alterations were more severe in marmosets with a fatal outcome. Similar histopathological alterations in the liver and biliary tract, except for ductal epithelial necrosis, have been previously reported in captive NHPs infected with P. illiciens (Sousa et al. 2008; Pereira et al. 2021; Oliveira et al. 2023).
Hepatic fibrosis, biliary epithelium hyperplasia, and duct proliferation were also the main pathological alterations observed in captive infected C. satanas (Pereira et al. 2021), as well as intraductal detection of trematode sections in free-ranging marmosets (Díaz-Delgado et al. 2018) and captive orangutans (Warren et al. 1998). Although the hepatic histopathological changes caused by P. illiciens parasitism in NHPs are well characterized, their functional impact on the liver and biliary tract remains unclear. In marmosets with HP, chronic weight loss and muscle mass depletion could be likely attributable to a catabolic imbalance related to the hepatic chronic inflammatory process and/or disruptions in some key liver metabolic pathways, leading to a progressive decline in overall physiological functions and ultimately contributing to a fatal outcome.
The high frequency and quantity of fluke eggs and trematode sections detected within the biliary tract of affected animals suggest a significant parasitic load in fatal cases observed in this study. An elevated trematode egg count in feces was associated with increased mortality, even after treatment with praziquantel in captive C. penicillata (Mati, Pinto, and de Melo 2021). Additionally, epithelial necrosis in the biliary tract, predominantly at the sites of fluke attachment, was substantially more significant in both captive and free-ranging marmosets with a fatal outcome. Secondary bacterial cholangitis and bacteremia, likely predisposed by the P. illiciens infection, were reported in a survey of HP cases in free-ranging marmosets (Oliveira et al. 2023). These findings suggest that P. illiciens parasitism, especially at higher intensities, may lead to significant hepatic damage and systemic alterations, resulting in progressive weight loss, cachexia, and death.
Morphological identification of hepatic trematodes in free-ranging and captive marmosets revealed primary body length, oral sucker size, pharynges, and a reproductive system with features compatible with P. illiciens (Pinto et al. 2017; Assis et al. 2021). Variations in the morphology and size of P. illiciens are considered frequent and are related to the stage of infection and host species (Pinto et al. 2017). Additionally, several previously identified fluke species are now considered to be morphological variations of P. illiciens infecting different hosts (Pinto et al. 2017; Pinto, Mati, and Melo 2016).
Given the high morphological variability and phenotypic plasticity within the Dicrocoeliidae family, molecular assays were necessary to determine the taxonomic position of fluke isolates accurately in this study, such as the cox1 gene as a molecular marker for species identification (González-García et al. 2020; Ranaraja et al. 2022). In the present study, phylogenetic analyses based on the cox1 nucleotide sequence (Pinto et al. 2018) supported the identification of all flukes within the pools as P. illiciens. As observed in this study, P. illiciens isolates from a cat, marmoset, lizard, and snail in Southwestern Brazil showed the mitochondrial cox1 sequences fully identical across all isolates, differing by only a single nucleotide (0.25%) from sequences obtained from a specimen collected from a cat in the Northeast region. The absence of pronounced host specificity suggests that the parasite's genetic composition remains relatively conserved across different hosts, resulting in minimal variability in the cox1 gene (Pinto et al. 2018).
The pathogenesis of HP in NHPs and other animal species remains undetermined; however, in other biliary tract trematodiasis such as fasciolosis, the mechanical damage caused by the parasite's migration and fixation, the release of parasitic products and an immune-triggered inflammatory reaction are associated with the hepatic tissue injury (Pérez-Caballero et al. 2018; Wesołowska et al. 2012; Mendes et al. 2010). Fasciola hepatica infections in ruminants have been linked to marked weight loss, likely due to increased protein turnover resulting from a protein/energy drain, appetite loss, and other factors (Parkins and Holmes 1989; Dargie 1987). The hepatic migration of flukes may enhance the release of inflammatory mediators such as cathepsin-B, cathepsin-L, and proline (Barbour et al. 2021; Corvo et al. 2009; Dalton et al. 2003). These molecules, along with other inflammatory modulators, appear to induce a heightened fibroblastic response, collagen production, and an immune response capable of damaging hepatic tissues (Pérez-Caballero et al. 2018; Mendes et al. 2010; Pérez et al. 2002). Since the pathogenesis of HP is still unclear, further studies analogous to those conducted on hepatic fascioliasis could provide valuable insights into the mechanisms of tissue damage and their effects on marmosets infected with P. illiciens.
Parasitism by P. illiciens can lead to significant gross and microscopic lesions, particularly in the gallbladder, bile ducts, and hepatic parenchyma, indicating that HP can be a fatal disease in free-ranging, but mostly in captive animals, rather than a minor or asymptomatic condition. Our findings suggest that chronic weight loss combined with high-intensity infections and severe hepatic lesions are strongly associated with the overall health decline of the animals and lethality observed in NHPs. Despite the overall advancements in understanding the disease presented in this study, the relationships among the sources and risks of infection, parasitic load and disease progression, as well as the broader impact of the disease on free-ranging NHP populations, remain to be elucidated. Even considering some knowledge gaps, epidemiological, clinical, and pathological findings in this study underscore the potential of HP to threaten free-ranging black-tufted marmoset populations in Central Brazil, a disease that has been neglected due to limited information.
Author Contributions
Isabel Luana Macêdo: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); Methodology (equal); validation (equal); writing–original draft (equal); writing–review & editing (equal). Davi Emanuel Ribeiro Sousa: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing–original draft (equal); writing–review & editing (equal). Antonizete dos Reis Souza: conceptualization (equal); data curation (supporting); investigation (supporting); methodology (supporting). Gabriela Rodrigues Toledo Costa: conceptualization (supporting); data curation (supporting); investigation (supporting). Marcela Corrêa Scalon: data curation (supporting); formal analysis (supporting); methodology (supporting). Matheus Almeida Duarte: conceptualization (equal); data curation (supporting); formal analysis (supporting); methodology (supporting). Giane Regina Paludo: conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); validation (equal). Estevam Guilherme Lux Hoppe: formal analysis (equal); methodology (equal); validation (equal). Wilson Junior Oliveira: data curation (supporting); formal analysis (supporting); methodology (supporting). Pedro Henrique Oliveira Passos: conceptualization (equal); formal analysis (equal); software (equal). Alessandro Pecego Martins Romano: conceptualization (equal); investigation (equal); methodology (equal); validation (equal). Eduardo Mauricio Mendes Lima: investigation (supporting); methodology (supporting); writing–original draft (supporting); writing–review & editing (supporting). Cristiano Barros Melo: conceptualization (equal); investigation (supporting); methodology (supporting); writing–original draft (supporting); writing–review & editing (supporting). Márcio Botelho Castro: conceptualization (lead); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); validation (equal); writing–original draft (lead); writing–review & editing (lead).
Acknowledgments
M.B. de Castro is a fellow of research productivity (PQ 1 C) granted by the National Council for Scientific and Technological Development, Brazil (grant number CNPq 307909/2021-2). We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES) for financing in part (Finance Code 001) the doctoral scholarship of Isabel L. de Macêdo and Davi E.R. Sousa.
Ethics Statement
As an Official Regional Laboratory for the Brazilian Ministry of Health for the diagnosis of Yellow Fever and other epidemic diseases in non-humannonhuman primates (NHPs), the Laboratory of Veterinary Pathology and Forensics (LVPF-UnB) routinely receives dead free-ranging NHPs for post-mortem examination and Yellow Fever testing. These animals are collected by State Health Surveillance Services (Federal District and the States of Goiás, Mato Grosso, and Tocantins) as part of the Brazilian National Program for Yellow Fever Control. This study utilized data and samples from the autopsy records archived at the LVPF-UnB. Therefore, ethical approval is not required for this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




