Temporal patterns of gut microbiota in lemurs (Eulemur rubriventer) living in intact and disturbed habitats in a novel sample type
Abstract
The gut microbiome is a plastic phenotype; gut microbial composition is highly variable across an individual host's lifetime and between host social groups, and this variation has consequences for host health. However, we do not yet fully understand how longitudinal microbial dynamics and their social drivers may be influenced by ecological stressors, such as habitat degradation. Answering these questions is difficult in most wild animal systems, as it requires long-term collections of matched host, microbiome, and environmental trait data. To test if temporal and social influences on microbiome composition differ by the history of human disturbance, we leveraged banked, desiccated fecal samples collected over 5 months in 2004 from two ecologically distinct populations of wild, red-bellied lemurs (Eulemur rubriventer) that are part of a long-term study system. We found that social group explained more variation in microbiome composition than host population membership did, and that temporal variation in common microbial taxa was similar between populations, despite differences in history of human disturbance. Furthermore, we found that social group membership and collection month were both more important than individual lemur identity. Taken together, our results suggest that synchronized environments use can lead to synchronized microbial dynamics over time, even between habitats of varying quality, and that desiccated samples could become a viable approach for studying primate gut microbiota. Our work opens the door for other projects to utilize historic biological sample data sets to answer novel temporal microbiome questions in an ecological context.
Highlights
-
Social group membership explains more variation in microbiome composition in red-bellied lemurs than individual host identity or site, even between two sites with different histories of human disturbance.
-
There are similar predictors of broad microbial traits in desiccated samples in our study as in ethanol-preserved samples in past studies.
Abbreviations
-
- ASV
-
- amplicon sequence variant
-
- LEfSe
-
- linear discriminant analysis effect size
-
- PC
-
- principal coordinates
-
- PCoA
-
- principal coordinate analysis
1 INTRODUCTION
Gut microbiome composition is temporally dynamic, and large gaps remain in our understanding of the causes and consequences of microbial change over an individual host's lifetime (Björk et al., 2019; Kolodny & Schulenburg, 2020). Wild animal systems provide a useful approach to model longitudinal microbiome dynamics, as they provide a series of “natural experiments” to characterize the relationship between changes in animals' social and physical environments, and corresponding shifts in their microbiota (Cusick et al., 2021; Davidson et al., 2020). For example, longitudinal wild animal microbiome studies could be used to provide insight into long-term environmental changes, habitat degradation, and climate change, as well as host life history-associated metrics (Björk et al., 2022; Grieneisen et al., 2021; McManus et al., 2021). Recent reviews have advocated for the importance of wild animals to the field of microbiome science, as well as emphasized the continued importance of longitudinal wild animal studies in general (Björk et al., 2019; Davidson et al., 2020; Grieneisen et al., 2023). However, linking the effects of long-term environmental change on gut microbiome dynamics of individual hosts requires many years of continuous data collections. As such, figuring out how to leverage already available longitudinal samples and host information in a microbiome context (i.e., by conducting microbial DNA extraction and sequencing on already banked samples) is a useful tool for placing our understanding of host-microbiome interactions in a long-term context.
Primates are a good candidate host taxon to fill this gap. Wild primate study systems often have lifetime data on long-lived animals; one review noted there are >95 long-term primate studies on more than 66 species (Chapman et al., 2017). These long-term studies often have databases of individual host-based data, as well as matched data on the animal's life history traits and physical and social environments. Furthermore, primates are good models for humans, and often have associated long-term environmental and social data that are not available in many human studies (Alberts & Altmann, 2012; Cox et al., 2013). Importantly for gut microbiome work, primate systems also often have banked fecal samples.
To date, a combination of banked and newly collected fecal samples have been used to test ideas about how primate microbiomes change over time. Temporal variation in primate microbiome composition and function has often been addressed in the context of seasonal environmental changes. A 4-year study in geladas (Theropithecus gelada) found microbiomes varied most strongly with rainfall and temperature (Baniel et al., 2021), and two studies on a 14-year data set in savannah baboons similarly found strong effects of rainfall (Björk et al., 2022; Grieneisen et al., 2021). Seasonal change in rainfall can also reflect changes in diet. Rainfall predicted overall microbiome composition in a several month study in white-faced capuchin monkeys (Cebus capucinus imitator), but specific taxa abundances fluctuated based on fruit versus arthropod availability (Orkin et al., 2019). Seasonal dietary shifts in fruit availability have even led to gut microbial and metabolomic convergence between two gorilla species (Gomez et al., 2016). Temporal variation in fruit and leaf consumption likewise led to corresponding shifts in metabolic potential in red-fronted brown lemurs (Eulemur rufifrons) (Murillo, Schneider, Fichtel, et al., 2022). Yearly fluctuations in food availability were posited to explain why year was the most important predictor of microbial composition in a study of Verreaux's sifaka, with members of the same social group demonstrating group-specific temporal shifts (Perofsky et al., 2021). However, it is worth noting that seasonal patterns are not always consistent between primate species; microbial alpha diversity is higher in the rainy season in savannah baboons (Papio cynocephalus) but highest in the dry season in red-fronted brown lemurs (E. rufifrons), suggesting the importance of different drivers between host species (Grieneisen et al., 2021; Murillo, Schneider, Fichtel, et al., 2022).
Longitudinal microbial variation in different quality habitats is not as well understood. Most studies that compare primate microbiota between sites are over limited temporal scales as they are cross-sectional by design (Amato et al., 2013; Bennett et al., 2016; McCord et al., 2013), or they do not report if collection season or year influenced their results (Barelli et al., 2015; Wasimuddin et al., 2022). Several studies have found a decrease in microbial diversity in low quality habitats (i.e., forest fragments or anthropogenically disturbed sites), including in the red colobus monkey (Procolobus gordonorum; [Barelli et al., 2015]), gray-brown mouse lemur (Microcebus griseorufus; [Wasimuddin et al., 2022]), and black howler monkey (Alouatta pigra; [Amato et al., 2013]). These differences can be explained in part by differences in diet quality between habitats (Amato et al., 2013; Barelli et al., 2015). However, the relationship between habitat quality and microbial diversity is not universal across primates. McCord et al. (2013) found little difference between colobus microbiomes in intact versus fragmented forest habitats (although they note that those in fragmented forest demonstrated greater individual variation). Similarly, Bennett et al. (2016) found that habitat disturbance did not affect microbial alpha diversity in ring-tailed lemurs (Lemur catta), while microbial compositional differences between habitats were driven by the presence/absence of rare taxa. Bornbusch et al. (2022) also did not find a distinct signature of captivity on the ring-tailed lemur microbiome, but consistent with McCord et al. (2013), found that captive lemurs had greater microbial variation than wild lemurs.
Longitudinal studies could help to explain this seemingly inconsistent relationship between habitat quality and microbiome composition and diversity across the literature. A 2-year study in the rufous mouse lemur (Microcebus rufus) between an intact and degraded site found that microbial richness was higher in the intact site in both years, but that Simpson diversity only differed between the sites in 1 year (Aivelo et al., 2016). Furthermore, the relationship between lemur age and microbial diversity differed between sites, suggesting that fine-scale longitudinal analyses are needed to tease apart the complex relationships between habitat quality, time, and host traits.
The long-term study populations of wild, red-bellied lemurs (Eulemur rubriventer) in Ranomafana National Park, Madagascar are especially well-suited for studying social and environmental effects on the microbiome over time. Red-bellied lemurs live in highly affiliative, cohesive family groups with no detectable hierarchy and almost no aggression (Overdorff & Tecot, 2006). Their social groups are stable, making them ideal for ecophysiological studies interested in detecting ecological signals (Tecot, 2008, 2013). This population in particular is also well-suited for conducting comparative ecological work because lemur social groups inhabit multiple distinct sites within the park that have different histories of deforestation and substantially vary in the availability of their preferred food source, fruit (Tecot, 2008).
In addition, banked samples are available for this system that were desiccated for long-term preservation of steroid hormones (using methods described in Tecot [2008, 2013] and Tecot et al. [2019]). Desiccated samples are frequently used in wild lemur studies and other primate field systems where immediate preservation in ethanol or freezing is not feasible. Samples initially collected and preserved following this approach for hormone analysis have later been used for host genetic analyses (Bradley et al., 2000; Chandrashekar et al., 2020; Diakiw, 2017; Jacobs & Bradley, 2016; Wasser et al., 1997), and naturally desiccated fecal samples (i.e., air dried without preservative media) have been used with varying degrees of success in other human and nonhuman primate microbiome studies (Bokulich et al., 2019; Grieneisen & Blekhman, 2018; Grieneisen et al., 2019; McDonald et al., 2018). Taken together, these studies suggest that analyzing desiccated fecal samples could be a promising technique for uncovering gut microbiome dynamics in extant historic data sets.
Past microbiome work on E. rubriventer within a single, primary forest site of this population has found strong signatures of family group membership on gut microbiome composition, with temporal variation in microbial diversity in relation to infant births (Raulo et al., 2018). These group-level distinctions persist when accounting for relatedness and diet (Whitney et al., accepted). However, it is unknown if these relationships differ between sites with varying levels of human-caused deforestation. The goal of our study therefore was to use desiccated samples to test if the effects of individual identity, social group, and time on gut microbiome composition and diversity differed between lemur populations living in habitats with different histories of human disturbance. We predicted that, similar to past work in other primate taxa (Amato et al., 2013; Barelli et al., 2015; Wasimuddin et al., 2022), lemurs at the sampling site with greater history of disturbance would have lower gut microbial diversity and different overall composition than those at the less disturbed site. We also predicted that, because red-bellied lemur social groups are small (typically consisting of one adult female, one adult male, and one to two of their offspring) and members of a social group engage in frequent social interactions (Overdorff & Tecot, 2006), temporal microbial patterns would be social group-specific, but would still vary between sites due to site-specific environmental differences (Tecot, 2008). Finally, we predicted that infant births would mark a significant increase in microbial dissimilarity between group members in both sites (Raulo et al., 2018).
2 METHODS
2.1 Study site
Ranomafana National Park is located in southeastern Madagascar, between 21̊ 02′–21̊ 25′ S and 47̊ 18′–47 3̊7′ E. It ranges from 500 to 1500 m in altitude, is dominated by submontane rain forest, and receives an average of 3090 mm of rain per year (Overdorff, 1991; Tecot, 2008; Wright et al., 2008). There is substantial interannual variation in total annual rainfall (1500–4000 mm), plant phenological patterns, and the lengths of wet and dry seasons (Tecot, 2008; Wright et al., 2005). We collected data from two ecologically distinct sites in the park; Talatakely and Vatoharanana. Talatakely was selectively logged in the late 1980s, was occupied before its designation as a national park in 1991, and consists of large areas of secondary growth (Wright et al., 2002). Detailed climate patterns and plant inventories and phenology in the two sites during the study period are described in Tecot (2008). Briefly, fruit in Talatakely was unpredictable from month to month, absent for prolonged periods, and not correlated with precipitation. Vatoharanana (approximately 6 km from Talatakely) experienced little logging and largely consists of primary growth. Fruit at this site was more predictable, with seasonal patterns, higher productivity, and shorter periods of scarcity.
2.2 Study population
We began studying these populations of red-bellied lemurs in 2000, following prior long-term studies (Durham, 2003; Overdorff, 1991). Here, we analyze samples from a study conducted between August 2003 and March 2005 in which we collected behavioral and fecal data from five social groups, three in Talatakely and two in Vatoharanana (Tecot, 2008). Each group consisted of a mated, adult pair and their offspring (Overdorff & Tecot, 2006). No infants were born in 2003, so we focus the current analyses on samples collected from August to December 2004, during which time all adult females gave birth. We distinguished individuals by variation in pelage color and patterns, body size, sexual dichromatism, and in some cases collars that were fit during other studies. We used the following age categories: adult (resident breeding pair) and non-adult offspring (including infant [0–1 yr], juvenile [1–2 yrs], subadult [2–3 or 4 yrs]).
2.3 Sample collection, preservation, and storage
We collected fresh fecal samples opportunistically from identified individuals during behavioral data collection for a separate project (aiming for 1–2 samples per lemur per week), with the help of field assistants. Because our initial study investigated glucocorticoid levels (Tecot, 2008, 2013), we collected samples before 1200 h to control for circadian variation in hormone excretion (Tecot et al., 2023). We only collected samples that were uncontaminated by urine and others' feces. Specifically, using latex gloves to handle samples, we put each sample into aluminum foil, and flattened and labeled each sample. We preserved samples via desiccation using the following procedure: We took samples back to the research station (Talatakely) or camp (Vatoharanana) within 4 h, and weighed and desiccated each sample. In Talatakely we placed samples in a drying oven at 70°C, and in Vatoharanana we dried samples by placing them on rocks next to a persistent fire, and transferred wet samples to the drying oven at the research station at the end of the week. We checked samples frequently and removed them once they were dry, discarded samples with mold, and stored dried samples in foil and Whirl-pak® bags (Nasco). We combined all bags into a larger Ziploc bag with desiccant. We kept all samples in dry storage conditions, stored at room temperature and at times in a −20 or −80 freezer (all samples treated the same). Our desiccation procedures (Tecot, 2008, 2013) have been used to preserve fecal samples in a range of species (Brockman et al., 1998; Tecot et al., 2019; Whitten et al., 1998; Ziegler & Wittwer, 2005).
2.4 Sample validation
Desiccated samples are a commonly banked sample type, but they have not yet been explored in detail for use in gut microbiome analyses. To validate their usage, we performed a pilot analysis using 16 desiccated samples and two positive control samples preserved in ethanol (see Supporting Information S1). Similar to past published work on this population using samples preserved in RNAlater (Raulo et al., 2018), we found that the most abundant phyla in both the desiccated and ethanol samples were Firmicutes (mean relative abundance = 44.5%), Bacteroidota (23.9%), and Proteobacteria (12.2%) (Supporting Information S1: Figure S1A). We also found that desiccated samples had a rough similarity to our positive ethanol controls in terms of amplicon sequence variant (ASV) richness (Supporting Information S1: Figure S1B), dominant families (Supporting Information S1: Figure S1C), dominant genera (Supporting Information S1: Figure S1D), and overall composition (PCoA; Supporting Information S1: Figure S1E). However, we note that the ethanol samples cluster separately from desiccated samples along Bray–Curtis PCs 3 and 4 (Supporting Information S1: Figure S1F) and that our sample size is too small to run a quantitative analysis that directly compares sample preservation media. Nonetheless, these pilot results were promising enough that we decided to move forward with a full study using desiccated samples.
2.5 Data set
To test how individual identity, social group, history of anthropogenic disturbance, and time predicted microbial diversity and composition, we used 159 desiccated fecal samples collected from 13 individual lemurs from August to December 2004 (i.e., 2–3 samples per individual per month for 5 months) from the two ecologically distinct sites. Each sample was from a known lemur individual, with corresponding data on age, sex, social group, sampling site, and collection date (Supporting Information S1: Table S2).
2.6 DNA extraction, sequencing, and quality filtering
We ground samples to a powder using a mortar and pestle, and sifted samples to remove seeds. We extracted DNA in the Laboratory for the Evolutionary Endocrinology of Primates using a MoBio POWERSOIL kit with standard protocol, less a minor modification of starting mass of 0.05 g of sample rather than the recommended 0.25 g, to account for the liquid absorbed by the dry matter. Illumina library preparation and 250 basepair paired-end sequencing of the V4 region of the 16S rRNA gene were conducted at the University of Minnesota Genomics Center. We included five blanks (four DNA extraction buffer blanks and one water blank) as negative controls.
We performed sequence processing, diversity calculations, and statistical analyses in R version 4.2.2 unless specified otherwise (R Core Team, 2021). Sequence processing and quality control were run using the DADA2 pipeline (Callahan et al., 2016). Reads were trimmed at 200F and 160R and quality filtered with expected error rate ee = 2. Taxonomy was assigned via the Silva v138.1 database (Quast et al., 2013). Contamination was checked using the package decontam (Davis et al., 2018).
After initial quality filtering, there were 10,002 ASVs. There were 63 ASVs flagged as potential contaminants by decontam, the majority of which (n = 36) were found in one blank sample and one real sample. Of the 63 ASVs, 56 were found in only one of the five blanks. The most common contaminant ASV (the only ASV found in 4 of the 5 blanks) mapped to Bradyrhizobium, an N-fixing soil bacterium often associated with plant roots. We removed all 63 ASVs flagged as potential contaminants, as well as ASVs matching to chloroplast or mitochondria, leaving 10,199 ASVs. We removed singleton ASVs (i.e., those found in only one sample), leaving 2302 ASVs. Across the 159 samples, 72% of reads were retained on average after quality filtering (5% of reads were retained in the negative control samples), leaving an average of 57,054 reads per sample (range: 3205–131,094) and an average of 937 reads (range: 722–1402) per negative control.
We calculated alpha diversity as ASV richness and Shannon's H in the vegan package (Oksanen et al., 2007). To normalize abundances, we performed cumulative sum square (CSS) transformation in the metagenomeSeq package (Paulson et al., 2013). To measure pairwise composition (i.e., beta diversity), we then calculated a Bray–Curtis dissimilarity matrix in vegan and ran a PCoA on the Bray–Curtis matrix. The first five principal coordinates (PCs) explained 32.1% of total variance (PC1: 16.6%, PC2: 5.6%, PC3: 3.9%, PC4:5 3.4%, PC5: 2.6%).
There was a strong correlation between read count and ASV richness (R = 0.89) and Bray-Curtis PC1 (R = 0.79), but not between read count and any metadata variables (p > 0.05 after correcting for multiple testing). This correlation was still strong even if we processed the data using a compositionality-aware metric (Aitchinson's PC1 on centered log-ratio transformed data and read count; R = −0.89). To account for these correlations, we (1) included read count in the alpha and beta diversity statistical tests and (2) repeated the analyses on a data set limited to n = 106 samples with at least 40,000 reads per sample, as informed by rarefaction curves (Supporting Information S1: Figure S2).
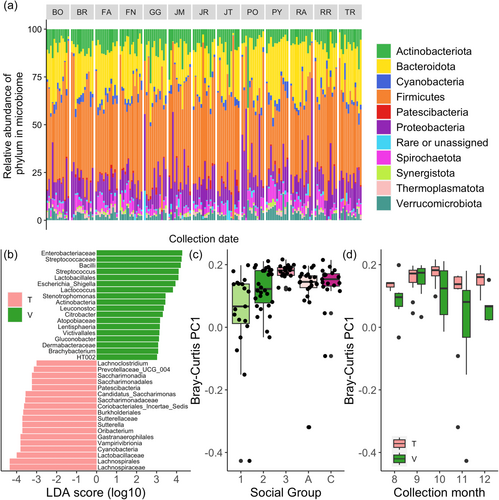
Because the 16 pilot samples and the additional 159 samples were sequenced using different read lengths (300 basepairs for the pilot samples vs. 250 basepairs for the 159 samples), the data sets were not combined for analysis. We note two additional differences in the library preparation and sequencing approach for the 16 pilot samples. First, an extra PCR cycle was run during library prep of the pilot samples, due to the low quantity of DNA. Second, pilot sample reads were trimmed at 250F and 200R, and quality filtered with an expected error rate of ee = 2.5 (see Supporting Information S1). Despite these differences, the 159 samples were qualitatively similar to the 16 pilot samples, with the same four phyla making up the majority of the reads; Firmicutes (mean relative abundance = 43.5%), Bacteroidota (20.6%), Proteobacteria (14.8%), and Actinobacteriota (9.0%) (Figure 1a), and the same three family categories comprising approximately one-third of the reads (Prevotellaceae, unassigned, and Lachnospiraceae; Supporting Information S1: Figures S1C and S3).
2.7 Statistical methods
To test for site-level differences in microbial alpha diversity, we ran linear mixed models using lmerTest with ASV richness and ASV Shannon's H as the response variables, host identity as a random effect, and read count, age, site, sex, social group (nested within site), and collection month as fixed effects (Kuznetsova et al., 2017). Sex and age were included because they have been included in gut microbial diversity models in past primate studies (e.g., Grieneisen et al., 2021; Raulo et al., 2018). To test if temporal variation in microbial diversity differed between sites, we also included a month (as numeric)*site interaction term. We used the drop1() function to pick the best model. Following Grieneisen et al. (2021), we repeated the linear mixed models with the first PC of the Bray–Curtis dissimilarity (PC1) as a response variable.
To test for site-level differences in microbiome composition, we ran PERMANOVAs using the adonis function in vegan, with read count, host identity, social group, site, age, sex, and month as fixed effects, and a Bray–Curtis dissimilarity matrix as the response variable.
To test for site-level differences in the relative abundance of specific microbial taxa, we used linear discriminant analysis effect size (LEfSe), following McManus et al. (2021). We ran LefSe at more stringent levels than the default settings, with Kruskal–Wallis set to alpha = 0.01 and an LDA effect size = 3.0 (Segata et al., 2011).
We next focused on temporal variation. To test if samples collected closer together in time were more similar than those collected further apart in time, as expected from microbiome samples of sufficient quality, we ran a mantel test in vegan on a pairwise matrix of microbial dissimilarity (Bray–Curtis) and a matrix of temporal dissimilarity (i.e., time difference between sample collection dates measured as days). We then used a partial mantel test to control for whether sample pairs were from the same or different hosts. To test if temporal autocorrelation varied by site, we repeated the partial mantel tests within each site.
Finally, we tested if microbial dissimilarity between group members increased after infants were born in both the primary forest site (following Raulo et al. [2018]'s results) and the site with greater human disturbance. To do so, we ran a Wilcoxon rank-sum test on Bray–Curtis values between individual hosts within the same family group across all groups, and then limited to the only family group (Group A; in the more disturbed site) that had similar numbers of prebirth and postbirth samples. Because of the read count correlations in the full data set, we only conducted this analysis on the data set limited to 106 samples.
3 RESULTS
3.1 Social group, site, individual identity, and sampling month predict overall microbial diversity and composition
We found mixed support for our prediction that temporal variation in individual host's microbiome composition would be stronger than social group effects, and that these effects would be environmentally dependent (i.e., differ by site). The best linear mixed model predicting Shannon's H included only read count, while richness was predicted by sex and social group, and Bray–Curtis PC1 was predicted by sex, with a site*sampling month interaction term that trended toward significance (p = 0.07; Supporting Information S1: Figure S4 and Tables S5 and S6). However, when we repeated the analysis on the data set limited to samples with >40,000 reads, we found that the best models for ASV richness and Shannon's H included read count alone, and that the best model for PC1 included social group and collection month (Figure 1C,D; Supporting Information S1: Table S7).
We initially found strong effects of time and individual host identity on microbiome composition. In the full data set, read count explained 10.7% of the variance, collection month explained 4.5%, and host identity explained 11.0% of the variance (PERMANOVA). However, host identity is nested within social group and site, and if those terms were also included in the model, site explained 2.6% variance, social group explained 3.8%, and host identity explained 4.6% but was no longer significant in the model (PERMANOVA; Supporting Information S1: Table S8). We saw similar but even stronger patterns in the reduced data set, in terms of collection month (8.0% of the variance), site (4.7%), social group (9.1%), and host identity (6.8%), with reduced effects of read count (2.7%) (PERMANOVA; Table 1).
| Df | Sums of squares | Mean squares | F model | R2 | Pr (>F) | |
|---|---|---|---|---|---|---|
| Read count | 1 | 0.440 | 0.440 | 3.396 | 0.027 | 0.001 |
| Site | 1 | 0.785 | 0.785 | 6.057 | 0.047 | 0.001 |
| Social group | 3 | 1.519 | 0.506 | 3.908 | 0.092 | 0.001 |
| Individual lemur identity | 8 | 1.130 | 0.141 | 1.090 | 0.068 | 0.146 |
| Collection month | 4 | 1.320 | 0.330 | 2.548 | 0.080 | 0.001 |
| Residuals | 88 | 11.399 | 0.130 | NA | 0.687 | NA |
| Total | 105 | 16.59 | NA | NA | 1 | NA |
- Note: Significant factors (p < 0.05) are in bold.
3.2 Taxa abundances vary somewhat by site
We expected that phyla abundances would differ between sites. However, Cyanobacteria was the only phylum that was differentially enriched, with greater relative abundance at Talatakely. In total, we found that 30 taxa at the level of genus through phylum varied in enrichment between sites (LEfSe; Figure 1b; n = 36 taxa in the reduced data set; Supporting Information S1: Tables S3 and S4). Three pathogen-associated genera (Citrobacter, Escherichia shigella, and Achromobacter) were enriched at Vatoharanana (the primary forest site) in both the full and reduced data sets.
3.3 Temporal variation
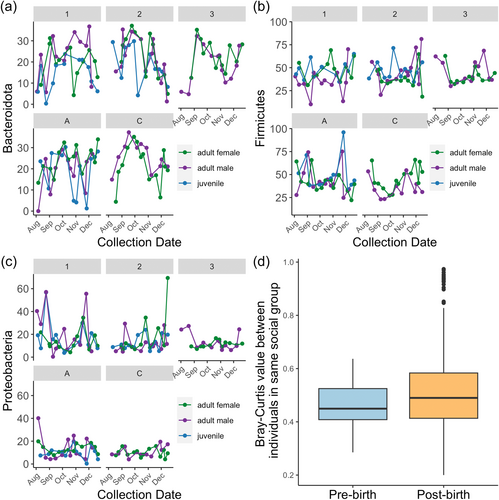
The top three most abundant phyla showed substantial temporal variation within individual hosts across social groups (Figure 2a–c). But, consistent with previous microbiome studies, we found that samples collected closer together in time were more similar (Mantel r = 0.146, p = 0.001; reduced data set r = 0.114, p = 0.001), even controlling for if samples were from the same of different individual hosts (partial Mantel r = 0.147, p = 0.001; reduced data set r = 0.114, p = 0.001). Partial mantels limited to within each site showed the same patterns (partial mantel reduced data set for Talatakely r = 0.1663, p = 0.003; for Vatoharanana r = 0.211, p = 0.003). Intra-group sample dissimilarity before versus after infants were born in the reduced data set showed similar trends to Raulo et al. (2018), but these differences were not statistically significant (Wilcox W = 9588, p = 0.067 Figure 2d; within Social Group A W = 565, p = 0.089).
4 DISCUSSION
4.1 Site differences in social, temporal, and ecological predictors of microbiome composition
Although forest sites differed in history of anthropogenic disturbance, overall composition and temporal variation in lemur gut microbial communities showed much stronger social group-specific than site-specific patterns. Specifically, even though sites differed in the abundance of some taxa compared to the less disturbed site, the sites did not differ in microbial alpha diversity. Additionally, social group and collection month were stronger predictors of overall gut microbial composition than site was (although we note that the positive trend in the site*sampling month interaction term suggests that future work should continue to explore differences in microbial temporal variation between environments).
We expected to find strong site effects in our data, especially given the differences in deforestation history as well as behavior and glucocorticoid levels (Tecot, 2008), but we note that past work has found mixed results on site differences in lemurs. Wasimuddin et al. (2022) studied lemurs at two sites and found that site had similar effects on the variance in microbiome composition as we found in the current study (site explained 3%–5% of the variance). However, they found an increase in disease-associated bacteria in the more disturbed site, which is not consistent with our results. Interestingly, in a study across four black-and-white ruffed lemur (Varecia variegata) populations (Donohue et al., 2019), only one site differed in microbial alpha diversity from the others, and another study in the same species only saw effects of individual identity in one of the three sites (McManus et al., 2021).
However, our results were consistent with past lemur studies in several key ways. First, our samples look like lemur samples; at a broad level, the three most abundant phyla (Bacterioidota, Proteobacteria, and Firmicutes) that we identified are the same as those found in E. rubriventer in RNAlater-preserved samples from one of the same sites almost 10 years later (Raulo et al., 2018). The same phyla were also found in a different ranked order in a recent study on ethanol-extracted samples in this species as well (Whitney et al., accepted). Bacteroidetes, Proteobacteria, Firmicutes, and our fourth most common phylum (Actinobacteriota) were also the most common taxa in a recent study on mouse lemurs (Wasimuddin et al., 2022). Second, factors that predict gut microbial composition in other primate studies were also important in our study. We found similar temporal effects as other primate microbiome studies; namely, that samples collected close in time are more similar than those collected further apart (Björk et al., 2022). We were also able to qualitatively replicate results from Raulo et al. (2018) that showed microbial similarity between social group members decreased after infants were born. We additionally make a novel contribution by showing that these patterns existed in multiple sites and with more frequent sampling from individuals, including a group with an out-of-season birth. Additionally, the strong social group effect that subsumed individual host identity in the PERMANOVA models is similar to results in Raulo et al. (2018) for E. rubriventer, and consistent with the dominant role of social group that Murillo, Schneider, Heistermann et al. (2022) found in red-fronted brown lemurs (E. rufifrons). Beyond primates, the dominance of social group over individual host identity has also been found in Egyptian fruit bats, where individuals have more similar microbiomes to the rest of their colony at a given timepoint than to themselves over time (Kolodny et al., 2019)
4.2 Utility and biases of desiccated samples
Many long-term studies in wild primates have decades of banked fecal samples. As such, figuring out how to leverage already available longitudinal samples and host information in a microbiome context (i.e., by conducting microbial DNA extraction and sequencing on already banked samples) is a useful tool for placing our understanding of host-microbiome interactions in a long-term context. Our results suggest that desiccated samples have the potential to be a viable, if imperfect, source of such long-term gut microbiome information, although we note that their potential needs to be more thoroughly studied in future work before we would advocate for their widespread use in primate microbiome studies.
There is some precedent for using drying approaches on gut microbiome samples. Past experimental work has shown that mammal samples left out for up to several days (i.e., naturally desiccated) yield microbial communities with minimal effects of exposure time on overall microbial composition and diversity in baboons (up to 48 h before preservation; Grieneisen et al., 2019); and minimal exposure time effects for up to a week in ungulates depending on the host species and local environment (Menke et al., 2015). These results have received mixed support from preservation methods comparative studies, which show that blooms of certain taxa are more common and larger compositional shifts happen over time compared to immediately preserved samples (Bokulich et al., 2019; McDonald et al., 2018; Pribyl et al., 2021; Song et al., 2016). Furthermore, combining multiple sample preservation types within the same analysis is not recommended, as varying preservation methods have biases towards certain taxa (although samples are often still identifiable to an individual host across preservation methods [Blekhman et al., 2016]). However, the American Gut Project, in which citizen-scientists dry-mailed fecal swabs for analysis, found that patterns of microbiome composition in preserved fecal samples were still evident in the dry swabs (Grieneisen & Blekhman, 2018; McDonald et al., 2018). They also found that the presence of blooms did not substantially affect study variables of interest, and that bloom-type taxa could be removed from analyses following dry-sample methods established by Bokulich et al. (2019). Taken together, these results suggest that analyzing desiccated fecal samples could be a promising technique for uncovering gut microbiome dynamics in extant historic data sets.
Desiccated samples could provide several advantages: they appear to yield microbiome results largely consistent with other microbial sample types based on limited pilot data, many are already banked and thus available for sequencing without additional field work required, and their availability opens the door to asking a range of questions about longitudinal microbiome change, especially when combined with environmental and host genetics data (Björk et al., 2019; Grieneisen et al., 2023). For example, we are now able to analyze the gut microbiome in additional samples spanning almost 2 years, for which we already have data on plant phenology, climate, behavior, reproduction, habitat quality, and glucocorticoids. We can then explore more specific predictors of the gut microbiome and how they vary across sites. Additionally, the increased flexibility in preservation that desiccated samples allow compared to other sample types means that their collection may make microbiome data collection feasible in field systems where it was previously thought to be impractical. This can be especially helpful in regions where populations may have gone extinct or declined such that gathering that information is now impossible. Finally, this work contributes to the emerging understanding of the potential importance of microbiomes in conservation applications (Ribas et al., 2023; Trevelline et al., 2019).
However, we note that there are limitations. Caution must be taken in interpretation of results from samples not immediately frozen or placed in preservative. The lower yield DNA than ethanol or other preservation methods means that DNA extraction and sequencing modifications, such as extra PCR cycles, may need to be used. Furthermore, the strong readcount effects mean that a higher number of samples may need to be excluded from analysis compared to other preservation methods, and a threshold beyond which readcount does not have an effect should be explored. However, we note that Donohue et al. (2019) had a maximum of 16,000 reads/sample in a study using ethanol-preserved samples from black-and-white ruffed lemurs, and their rarefaction curves did not reach a plateau. Past studies have shown that dry-preserving is more prone to blooms of certain taxa than ethanol-preserving (Bokulich et al., 2019), which, although not evident in our study, is an important consideration for future work. Furthermore, different microbial taxa may be more or less susceptible to degradation under different preservation methods, such that finer level taxonomic analyses may be limited in desiccated samples (Amir et al., 2017; Marotz et al., 2021). This indeed may be the case in our data set; 32.8% of reads were not identified at the genus level. However, we note that other lemur work has found high levels of unidentifiable genera. For example, a study of red-fronted brown lemur samples preserved in RNA later yielded a data set where 12 of the 20 most abundant microbial genera couldn't be classified below the family level, including the top four most abundant genera (which made up 34% of the reads on average [Murillo, Schneider, Fichtel, et al., 2022]).
To provide quantitative support for desiccation as a viable microbiome preservation medium, a pair-matched study (i.e., the same fecal sample aliquoted between desiccated and ethanol preservation techniques) should be run. This would be especially important for understanding which taxa differ in abundance between preservation techniques. A future comparative methods study should also be run to look at whether the different drying techniques used between the sites could be responsible for the inter-site taxonomic differences we found. We additionally recommend that other researchers conduct paired aliquot analyses in their own study systems to confirm system- and methods-specific results.
4.3 Future directions
We conclude with one final, important implication of this study. If microbial DNA can be extracted and sequenced from samples that are preserved using desiccation, it also opens the door to modeling longitudinal relationships between gut microbiome metrics and hormone levels in cases where samples are unable to be cryopreserved. Paired hormone–microbiome samples would be available from archived samples for many primate systems, including the lemur system used in the current study. Linking hormone and microbiome dynamics is important because the endocrine system has complex interactions with the microbiome via the gut-brain axis, with consequences for host health (Dinan & Cryan, 2017; Slevin et al., 2020; Tetel et al., 2018). Very few studies to date have tested the relationship between the gut microbiome and hormone levels in wild animals, and even fewer have had the paired longitudinal sample data to look over extended periods of time (Mallott et al., 2020; Stothart et al., 2019; Vlčková et al., 2018). Similar to host-associated microbial communities (Kolodny & Schulenburg, 2020), hormone levels are both plastic and temporally dynamic (Petrullo et al., 2022), such that paired longitudinal studies could answer questions about environmental stress, developmental cues, and microbially-mediated behavior mechanisms.
AUTHOR CONTRIBUTIONS
Laura Grieneisen: Conceptualization (equal); formal analysis (lead); visualization (equal); writing—original draft (lead); writing—review and editing (equal). Allison Hays: Data curation (equal); formal analysis (equal); methodology (equal); writing—review and editing (equal). Erica Cook: Data curation (equal); formal analysis (equal); methodology (equal); writing—review and editing (equal). Ran Blekhman: Methodology (equal); resources (equal); software (equal); writing—review and editing (equal). Stacey Tecot: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); resources (equal); writing—original draft (equal); writing—review and editing (equal).
ACKNOWLEDGMENTS
We acknowledge the communities that inhabit the area around Ranomafana National Park, and previously inhabited land inside the park boundaries, and we are grateful to have received permission to conduct this research. We thank the Malagasy government, Association Nationale pour la Gestion des Aires Protègèes (now Madagascar National Parks), Ministère des Eaux et Forêts, the Université de Madagascar, Madagascar Institute pour la Conservation des Ecosystèmes Tropicaux, Institute for the Conservation of Tropical Environments, and Centre ValBio for permissions and logistical support in Madagascar. We are particularly grateful for the help we received from Razafiarimalala Aimee, Adriamihaja Benjamin, Patricia Wright, Deborah Overdorff, and Jean Phillip Puyravaud. We thank field assistants Tricia Calhoon, Alex Hall, Rakotonirina H. F. Laingoniaina, Telo Albert, Rasendry Victor, Rakotonirina Thierry Emile, and the late Rakotoniaina Jean Felix for help and care collecting data, and Rakotonirina Michel for maintaining camp and sustaining all of us in the field. This material is based upon work supported by the National Science Foundation under Grant #BCS-0424234 (ST). This research was also supported by ASPIRE funds at the University of British Columbia-Okanagan (LG). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. This work was also supported by Primate Conservation, Inc., Conservation International Primate Action Fund, American Society of Primatologists, the University of Texas-Austin, and the TPW Foundation. We are also grateful for the helpful comments of the editor, reviewers, and Karen Bales, and discussions with members of the Grieneisen Lab, Blekhman Lab, and the Laboratory for the Evolutionary Endocrinology of Primates.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
E. rubriventer is classified as Vulnerable to extinction (Irwin et al., 2021). This research followed the ASP Code of Best Practices for Field Primatology. It did not involve capture or handling of the study subjects, and study subjects were re-habituated before study after having been the subject of prior research for approximately 15 years (e.g., Durham, 2003; Merenlender, 1993; Overdorff, 1991). This research adhered to (1) the legal requirements of Madagascar in which the research was conducted, under permit #143/MINENV.EF/SG/DGEF/DPB/SCBLF/RECH, (2) the protocols approved by the University of Texas at Austin Institutional Animal care and Use Committee, protocol #04032601, and (3) the American Society of Primatologists (ASP) Principles of the Ethical Treatment of Non-Human Primates.
Open Research
DATA AVAILABILITY STATEMENT
Raw sequencing reads are available in the NCBI SRA archive (BioProject PRJNA1109416; https://www.ncbi.nlm.nih.gov/sra/PRJNA1109416). R code and sample metadata files needed to reproduce the main text figures and major statistical analyses are available on Zenodo (https://zenodo.org/doi/10.5281/zenodo.11105895).




