The role of sleeping sites in the predator-prey dynamics of leopards and olive baboons
Abstract
Predation is widely recognized as an important selective pressure on prey animals such as baboons (Papio spp.), which face high leopard (Panthera pardus) predation risk, particularly at night. Baboons regularly sleep on cliff faces and in trees at night, ostensibly to avoid such predators. Despite retreating to such “refuges,” baboons are most often killed by leopards at or near their sleeping sites. Because of the challenges of studying nocturnal behavior and human-averse predators, few systematic data exist to reveal how leopard ranging near baboon sleeping sites influences baboons’ selection of sites and behavior at those sites. To investigate leopard-baboon dynamics at sleeping sites we deployed GPS/VHF radio collars on six representatives of four baboon groups and four leopards during a 14-month field study in Kenya. We used locations recorded every 15 min to identify baboons’ cliffside and riverine sleeping sites, the frequency and duration of leopard visits to these sites, and baboons’ adjustments in site use after leopard visits. Collared leopards visited riverine sites more frequently than cliffside sites, whereas most baboon groups strongly preferred cliffside sites, suggesting that leopard visits were often due to factors other than baboon presence, and that baboons used cliffside sites to reduce their risk of leopard predation. Regardless of type, collared leopards remained near baboon-occupied sleeping sites longer than vacant ones, indicating interest in hunting baboons then. Baboons at riverine sites departed later on mornings after leopard visits. Baboon groups occasionally shared sleeping sites simultaneously, possibly reducing risk through dilution. However, they did not reduce risk by frequently changing sleeping sites, minimizing detection at sleeping sites, or after leopard visits, arriving earlier the next evening or moving to a different site. Future research should explore if baboons readily detect nocturnal leopard presence and if predation-related changes in sleeping site use have cascading ecological effects.
1 INTRODUCTION
As a major selective force, predation is thought to have favored the evolution of numerous antipredator adaptations, including securing safe sleeping sites (Anderson, 1984, 1998; Hamilton, 1982). Baboons (Papio spp.) are well known for using cliffs, rocky outcroppings, and tall trees as sleeping sites (e.g., Altmann & Altmann, 1970; Cowlishaw, 1994, 1997; Hamilton, 1982; Markham, Alberts, & Altmann, 2015; Schreier & Swedell, 2008; Whiten, Byrne, & Henzi, 1987). Spending the night in such elevated locations is likely to be safer than spending the night on the ground in environments where mammalian predators such as leopards (Panthera pardus), lions (P. leo), and spotted hyenas (Crocuta crocuta) also live (Altmann & Altmann, 1970; Anderson, 1984, 1998; Busse, 1980; Buxton, 1951; Cheney et al., 2004; Cowlishaw, 1994; DeVore & Hall, 1965; Hamilton, 1982). Because such sites are spatially clumped and limited resources, they can influence both ranging behavior (DeVore & Hall, 1965; Hamilton, 1982; Anderson, 1984; Strandburg-Peshkin, Farine, Crofoot, & Couzin, 2017) and intergroup interactions (Altmann & Altmann, 1970; Markham, Guttal, Alberts, & Altmann, 2013; Markham et al., 2015). Limited numbers of sleeping sites may also create prey hotspots for predators (Emsens, Hirsch, Kays, & Jansen, 2014). In fact, most documented predation events on baboons have occurred at or very near sleeping sites (Busse, 1980; Cheney et al., 2004; Cowlishaw, 1994; Isbell, Bidner, Van Cleave, Matsumoto-Oda, & Crofoot, 2018; Matsumoto-Oda, 2015). It has been hypothesized that baboons may have in turn evolved additional antipredator strategies to reduce the risk of nocturnal predation, such as frequently changing sleeping sites, moving to a different sleeping site after an attack, temporarily increasing group size by sharing the same sleeping site with other groups, or, following nights when predators are detected, arriving earlier at, or departing later from, sleeping sites (Altmann & Altmann, 1970; Anderson, 1984; Hamilton, 1982; Markham et al., 2015; Matsumoto-Oda, 2015; Schreier & Swedell, 2012).
Leopards are the main predators of baboons (Busse, 1980; Cheney et al., 2004; Cowlishaw, 1994). A meta-analysis found that leopards eat baboons significantly less often than expected based on prey abundance (Hayward et al., 2006), although at some times in some places leopard predation may be intense. For example, in the Waterberg Mountains of South Africa, baboons constituted over 20% of identified prey in the diet of three female leopards (Jooste, Pitman, van Hoven, & Swanepoel, 2012; Jooste, Hayward, Pitman, & Swanepoel, 2013). Leopards are solitary hunters that inhabit many types of environments in Africa and Asia, resulting in widely varying activity patterns and prey spectra (Hunter et al., 2013). They have been found to be more active at night in more open woodland habitats than in heavily forested habitats (Bailey, 1993; Martins & Harris, 2013; Jenny & Zuberbühler, 2005). As solitary hunters, leopards prioritize hunting success by biasing activities to time periods and habitat types (Balme et al., 2007) when prey capture is most likely, and focus on prey species that are least likely to cause debilitating injuries (Hayward et al., 2006).
While the body mass of baboons, at more than 20 kg for adult males, is within the preferred prey mass of leopards (Hayward et al., 2006; Jolly, 2013), baboons typically live in larger groups than preferred leopard prey, and have been documented to chase, injure, and even kill leopards during the day, which may account for both their status as non-preferred prey (Hayward et al., 2006), and the nocturnal bias in leopard predation on baboons (Busse, 1980; Cheney et al., 2004; Cowlishaw, 1994; Isbell et al., 2018). Despite their use of ambush hunting tactics to surprise and kill prey, including primates (e.g., Cowlishaw, 1994; Isbell et al., 2018; Jenny & Zuberbühler, 2005), leopards hunted baboons at sleeping sites at night in Botswana without the element of surprise (i.e., using “pursuit hunting” tactics), which Busse (1980) suggested was due to the lack of harassment by baboons to leopards at night.
Most documented cases of leopard predation on baboons have occurred as a result of observers witnessing leopard attacks or finding the remains of baboons with leopards nearby (e.g., Cheney et al., 2004; Cowlishaw, 1994; Matsumoto-Oda, 2015). However, this provides only snapshots in time and space of predation and antipredator behavior. To more fully understand predator-prey relationships, their interactions must be investigated as a dynamic process of decisions made by both predators and prey. Unfortunately, investigating nocturnal predator-prey interactions has been difficult because humans are naturally diurnal with limited vision at night and leopards tend to avoid humans, including those who study primates on foot (Isbell & Young, 1993). To overcome these limitations, we used GPS/VHF radio collars placed on leopards and olive baboons (P. anubis) to remotely monitor their movements over a 14-month period in Laikipia, Kenya. Using that technology, we previously found that all known and suspected cases of leopard predation on baboons at our study site occurred at or very near their sleeping sites at night or around dawn (Isbell et al., 2018). We thus now focus on predator-prey interactions specifically at baboon sleeping sites.
Here we address the following questions: 1) Do characteristics of sleeping sites (cliff faces and tall trees, hereafter called “cliffside” and “riverine” sleeping sites, respectively), presence or absence of baboons, or phases of the moon influence decisions by leopards to visit baboon sleeping sites at night? 2) Does the presence of baboons affect the duration of time leopards spend at baboon sleeping sites? 3) Do baboons express behaviors proposed to be antipredator adaptations? In particular, do they choose highly inaccessible sleeping sites; routinely change their sleeping sites, move to different sites, depart later from sleeping sites, or arrive earlier at subsequent sleeping sites after leopards visit; attempt to avoid detection by being quiet at night or choosing sleeping sites that have extensive dense vegetation in which to hide; or dilute individual likelihood of predation by sharing sleeping sites with other baboon groups?
2 METHODS
2.1 Study site
We conducted field research at Mpala Research Centre (MRC) on the Laikipia Plateau of central Kenya (0.29°N, 33.90°E) from December 2013 to January 2015. The region is semi-arid; rainfall for MRC in 2014 was 443.2 mm. MRC supports a wide diversity of carnivorans, including an estimated 31 leopards, and their prey (Augustine & McNaughton, 2004; O'Brien & Kinnaird, 2011; T. O'Brien. pers. comm.). In our 130 km2 study area largely within MRC, leopard trapping and camera traps allowed us to identify 15 individual leopards: 2 fully adult males, 5 subadult males, 5–6 adult females, 1 subadult female, and 1 dependent cub. There were also others that we could rule out as any of the above 15, but we could not confidently identify them individually. The study area includes riverine habitat along the Ewaso Nyiro River dominated by Acacia xanthophloea trees, and wooded grasslands away from the river dominated by the shrubs A. etbaica, A. mellifera, and A. brevispica, and the tree Boscia angustifolia. See Q and Young, Okello, Kinyua, and Palmer (1998) for additional ecological information about MRC.
2.2 Study subjects, capture procedures, and data collection
In January 2014 we caught six adult female olive baboons from four groups (Table 1) in pre-baited, hand-triggered cage traps placed near regular sleeping sites (Jolly, Phillips-Conroy, & Mueller, 2003), and immobilized them with 10 mg/kg ketamine (Sapolsky & Share, 1998). We attached GPS/VHF radio collars (Savannah Tracking, Nairobi, Kenya) onto each female. Following collar attachment, each baboon was returned to a cage trap for safe recovery, and released when fully ambulatory.
| Species | Group (group size) | Individual (sex) | GPS collar dates (mo/dy/yr) |
|---|---|---|---|
| Panthera pardus | n/a | Konda “KO” (F) | 1/20/14–4/18/14 |
| 7/21/14–1/27/15 | |||
| n/a | Haraka “HA” (F) | 1/24/14–4/28/14 | |
| 7/16/14–1/19/15 | |||
| n/a | Chumvi “CH” (F) | 1/21/14–5/25/14 | |
| n/a | Tatu “TA” (M) | 3/29/14–7/11/14 | |
| Papio anubis | AI (61) | Wanga (F) | 1/19/14–10/10/14 |
| AI (61) | Yakobo (F) | 1/19/14–1/15/15 | |
| LI (59) | Luna (F)a | 1/15/14–6/29/14 | |
| LI (59) | Thelma (F)b | 1/15/14–6/7/14 | |
| ST (29) | Msafiri (F) | 1/16/14–1/27/15 | |
| MG (46) | Shujaa (F) | 1/23/14–1/8/15 |
- a Disappeared during the study.
- b Died from leopard (Tatu) predation.
We trapped three adult female leopards and one subadult male leopard (Table 1) using modified foot snare trap sets (Frank, Simpson, & Woodroffe, 2003), and immobilized them with a combination of ketamine and medetomidine (5 mg/kg and 0.05 mg/kg, respectively). We attached GPS/VHF radio collars onto each leopard. Following these procedures, we administered the reversal agent antipamizole (0.6 mg/kg) to each leopard, and stayed with them until they were ambulatory.
All collars were programmed to take GPS fixes synchronously every 15 min continuously throughout the life of the collars. The lifespans of the collars ranged from 3 to 14 months. Two baboon collars became nonfunctional when two females from the same group died 6 months into the study (one of whom was killed by a collared leopard: Isbell et al., 2018), and another baboon collar failed after 10 months, but all other baboon collars remained functional until the end of the study. Two leopard collars failed after 3 months and were removed but not replaced because the subadult male leopard (TA) had begun to disperse, and one collared female (CH) had little home range overlap with the primate groups. The two other leopard collars also failed after 3 months but were replaced within 3 months with collars that lasted until the end of the study.
We downloaded data from GPS/VHF collars using a base station (e-obs GmbH, Gruenwald, Germany) and two types of antennae (omnidirectional marine antenna, cxl 900-3LW: Procom, Frederikssund, Denmark; nine element Yagi antenna, YAGI-869A: Low Power Radio Solutions, Witney, United Kingdom) when we were within UHF range of each collar. We downloaded the data at least once per week whenever possible. Home ranges of the baboon groups were 2860–7061 ha whereas home ranges of individual leopards were 1753–3492 ha, based on 96.5 and 97.2% successful location fixes, respectively (Isbell et al., 2018).
We recorded vocalizations at the Hippo Pool sleeping site with an acoustic recorder (Songmeter SM2; Wildlife Acoustics, Maynard, MA) positioned in the center of the sleeping site. We configured it to record at 48,000 MHz sampling frequency from 17:00–08:00 every night. It recorded in separate 1-h digital audio files all vocalizations at the sleeping site (for details see Isbell and Bidner, [2016]).
2.3 Ethical note
The procedures in this study were approved by the Institutional Animal Care and Use Committee at the University of California, Davis (IACUC protocol # 17477) and by the Kenya Wildlife Service. These procedures adhere to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Non-Human Primates.
2.4 Data analyses
We identified sleeping sites based on clusters of baboon GPS locations from the same individual less than 50 m apart within the hours of 18:00–05:45 using Google Earth Pro v. 7.1.5.1557. We then determined occupancy of sleeping sites by baboon groups for every night when at least one group member had a functional GPS collar. We used the location of the collared group member with the most uninterrupted GPS fixes at 23:00 local time in Kenya (3 + UTC), as the location of the group. We operationally defined the time of departure from the sleeping site each morning as the time of the first collar location at least 50 m from the sleeping site after which subsequent locations were also 50 m or farther from the site, and the arrival at the sleeping site each evening as the first time within 50 m of the site after which all subsequent nocturnal locations were also within 50 m of the site. We defined collared leopard visits to sleeping sites during nocturnal (18:00–05:45) and diurnal (6:00–17:45) ranging as all leopard movements to within 160 m of a sleeping site. The distance of 160 m is the approximate mid-point of the mean group diameter per second over 14 days for one baboon group (MG) in which most adults were collared for a different study (58.9 m ± 37.6 SD perpendicular and 67.8 m ± 45.1 SD parallel to the direction of travel) (Crofoot, Kays, & Wikelski, 2015), and in known cases of leopard predation, leopards began their approaches well before they were 200 m from the primates (Isbell et al., 2018). We estimated the duration of each nocturnal leopard visit to a sleeping site by summing all 15-min intervals between GPS locations within 160 m of the site. We excluded four visits during trapping procedures that likely extended the duration of leopard proximity.
Although sunset in the study area occurred near 18:30 in all months (2014 mean sunset time = 18:36, range 18:19–18:50, http://aa.usno.navy.mil/data/docs/RS_OneYear.php), we included leopard locations starting at 18:00 into our analysis of “nocturnal” ranging here because the mean arrival time of the baboon groups at sleeping sites occurred at 17:58 (±55.7 min SD, N = 1236 group-nights, Table 2), and began the “diurnal” period at 06:00 as the earliest departures by baboons from sleeping sites occurred at 06:00. Nocturnal and diurnal time periods used here differ slightly from those used in Isbell et al. (2018) due to our specific focus on sleeping site use. In order to determine moon phase for nights during leopard visits we used the fraction of the moon illuminated at midnight from the US Naval Observatory website (http://aa.usno.navy.mil/data/docs/MoonFraction.php) and categorized moon phase as full (>0.75), quarter to three-quarter crescent (0.25–0.75), and new (<0.25).
| Group | Na | Main site N (%) | Mean arrival time (range)b | Mean consecutive nights (range) | Shared nights Nc |
|---|---|---|---|---|---|
| AI | 361 | 302 (84) | 17:52 (16:00–19:45) | 7.2 (1–59) | 17 |
| LI | 165 | 104 (63) | 18:33 (16:30–19:30) | 3.8 (1–25) | 28 |
| ST | 377 | 191 (51) | 18:24 (15:00–20:30) | 2.4 (1–13) | 44 |
| MG | 350 | 155 (44) | 17:22 (14:15–21:15) | 2.1 (1–11) | 3 |
- a N: Number of nights of GPS/VHF collar data from at least one group member.
- b Time at which collared group member was within 50 m of sleeping site on nights with no missing data around arrival time N = 347 (AI), 162 (LI), 366 (ST), 341 (MG).
- c Shared nights: Number of nights on which the group shared a sleeping site with another collared group.
For sleeping sites along the Ewaso Nyiro River, we quantified the number of trees in the grove(s) used by baboons at each site, and recorded the presence or absence of vines on each tree for their potential to hide baboons from leopards and/or hamper the ability of leopards to access baboons in such trees.
We estimated home ranges for all collared animals using the classic kernel method determined based on 99% of GPS locations that we uploaded to Movebank (www.movebank.org) via the R package “adehabitat” (Calenge, 2006). Locations of baboon sleeping sites relative to leopard home ranges were then assessed using Google Earth Pro v. 7.1.5.1557.
We used Chi-square goodness of fit and association tests to compare leopard visits to cliffside and riverine sites based on baboon presence or absence, moon phase, and the effects of leopard visits on site use by baboons. We used Mann–Whitney U tests to compare the duration of leopard presence at sleeping sites by type and baboon presence/absence, and Kruskal–Wallis tests to compare the duration of leopard presence at sites based on moon phase. We also used Mann–Whitney U tests to compare baboon groups’ departure and arrival times at cliffside and riverine sites following collared leopard visits.
We used the group-night as the unit of analysis for baboons. While it can be argued that this violates statistical rules of independence (as can any behavioral sequence involving individuals or groups within a population), we believe that this is used appropriately here because each night was separated by 12 hr of daylight and within that time, each of our four baboon groups had access to multiple sleeping sites, and could move in any of many potential directions relative to where they slept the night before, that is, where they slept each night was likely determined at least as much or more by where they moved in the preceding 12 hr than where they slept the night before (e.g., Markham et al., 2013; Pebsworth, Macintosh, Morgan, & Huffman, 2012; Shreier and Grove, 2014). Nights or group-nights are also common units of analysis in other studies of nocturnal behavior in diurnal primates (e.g., Markham et al., 2013; Pruetz, 2018; Tagg et al., 2018). We used sleeping site visits as the unit of analysis for leopards. We believe this is also used appropriately because visits by each of the leopards to any of the baboon group sleeping sites were spaced apart by 1–67 nights.
Analyses were run in SYSTAT (Version 13) and Vassarstats (http://vassarstats.net) with significance set at p = 0.05.
3 RESULTS
3.1 General characteristics of baboon sleeping sites
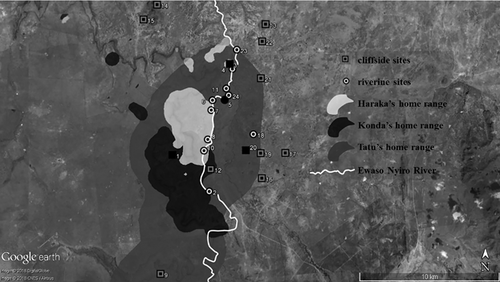
We identified 24 sleeping sites based on locational data from 1253 baboon group-nights. Of the 24 sleeping sites, 13 were cliffside sites, consisting of steep, rocky ledges or faces of cliffs, or rocky outcroppings (“kopjes”), and 11 were riverine sites, consisting of relatively tall A. xanthophloea tree groves along the Ewaso Nyiro River or along the edge of a lake (Figure 1, Table 3). “Baboon Cliffs,” the most frequently used cliffside site, rises 22.5–38.0 m between the low-lying surroundings and the escarpment on Mpala (Matsumoto-Oda, 2015) (Figure 1, Table 3). The number of trees varied widely at riverine sites (range: 7–42, mean: 18.4), as did the percentage of trees with vines (range: 0–38%; mean: 17.8%). The majority (70%, N = 7) of riverine sites had trees with branches overhanging the river that could facilitate crossing.
| Use by baboon groupsb | |||||
|---|---|---|---|---|---|
| Site name (Map #) | Consecutive nights, mean (range)a | AI | LI | ST | MG |
| CLIFFSIDE SITES | |||||
| Baboon Cliffs (1) | 13.8 (1–59) | 302 | 0 | 19 | 0 |
| Clifford's Kopje (3) | 3.1 (1–13) | 0 | 0 | 1 | 155 |
| Jessel (9) | 1.5 (1–2) | 3 | 0 | 0 | 0 |
| Mlima Fisi (12) | 1 | 1 | 0 | 1 | 0 |
| Mt. Doom (13) | 1.4 (1–2) | 0 | 0 | 0 | 10 |
| Mukenya (14) | 3.3 (1–15) | 29 | 0 | 0 | 4 |
| Mukenya Glade (15) | 2.8 (1–5) | 11 | 0 | 0 | 0 |
| OJ Airstrip (16) | 1.3 (1–2) | 0 | 0 | 10 | 0 |
| OJ East (17) | 1 | 0 | 0 | 3 | 0 |
| OJ Main house (19) | 2.5 (1–9) | 0 | 0 | 95 | 0 |
| OJ Mlima (20) | 3.4 (1–18) | 0 | 48 | 191 | 1 |
| OJ North (21) | 2.7 (1–6) | 0 | 5 | 0 | 56 |
| OJ North Mlima (22) | 1.8 (1–5) | 0 | 0 | 0 | 43 |
| Total | 346 | 53 | 320 | 269 | |
| RIVERINE/LAKESIDE SITESc | |||||
| Campsite (2) | 1 | 1 | 0 | 0 | 0 |
| Clifford's River (4) | 1.7 (1–4) | 0 | 3 | 0 | 44 |
| Corner (5) | 5.5 (1–25) | 0 | 104 | 0 | 0 |
| Eagle (6) | 1.4 (1–3) | 0 | 1 | 10 | 0 |
| Fig (7) | 2.2 (1–4) | 0 | 0 | 26 | 0 |
| Hippo Pool (8) | 1.7 (1–4) | 0 | 3 | 8 | 0 |
| Johanna (10) | 1.3 (1–3) | 14 | 0 | 12 | 0 |
| Meg's (11) | 2.1 (1–8) | 0 | 0 | 0 | 36 |
| OJ Lake (18) | 1 | 0 | 0 | 1 | 0 |
| River Bend (23) | 1 | 0 | 0 | 0 | 1 |
| School (24) | 1 | 0 | 1 | 0 | 0 |
| Total | 15 | 112 | 57 | 81 | |
- a Consecutive nights during the study at which each sleeping site was used by one or more baboon study group.
- b Number of nights group occupied each sleeping site with main sleeping site numbers in bold.
- c All sites below are located along the Ewaso Nyiro River except OJ Lake, which is located near the margin of a lake.
Thirteen of the 24 (54%) sleeping sites were only ever used by one of our study groups, 10 (42%) were used by two groups on the same or, more commonly, on alternating nights, and one (4.2%) was used by three different groups though no more than two groups shared the site on the same night (Table 3). The four baboon study groups each used 7–12 sleeping sites during the study, (Table 3) but each group preferred one particular “main” sleeping site, using it on 44–84% of all nights (Table 2).
All groups used both cliffside and riverine sites (Table 3) but cliffside sites were used more often (79% of 1253 group-nights, N = 988) than riverine sites (21% of group-nights, N = 265). Only the group with a riverine sleeping site as their main sleeping site (LI Group) used riverine sites more (68% of 165 nights; N = 112) than cliffside sites. Although the home range of the LI Group included a longer portion of the Ewaso Nyiro River (6.8 km straight-line distance vs. 5.6–5.9 km for the other three groups, Figure 1), two of the other three groups (AI and MG) did not sleep at all of the riverine sites that fell within their home ranges, indicating that their access to riverine sleeping sites was not limited. Similarly, LI Group did not have limited access to cliffside sites; five cliffside sites fell within their home range, but they slept only at two (Figure 1, Table 3).
3.2 Nocturnal visits by leopards to baboon sleeping sites: Site type preference and baboon presence
Eighteen of the 24 baboon sleeping sites were located within the home ranges of the collared leopards, including all 11 riverine sites (Figure 1). One leopard (CH) did not visit any sleeping sites used by baboon groups in this study, whereas the other three visited them a total of 100 times at night. Of those visits, 87% were to riverine sites (N = 87). Baboons were rarely at riverine sites (8%, N = 7 when leopards visited), suggesting that leopard movements along the river were largely independent of baboons as prey. In contrast, because baboons used cliffside sites more frequently than riverine sites, they were usually present on the rarer nights when leopards visited cliffside sites (85%; 11 of 13 nights) (Figure 2), suggesting that baboons could be reliably found as prey at night, and thus that cliffside sites were prey hotspots. We found, however, that leopards did not take advantage of this to go to baboon-occupied cliffside sites more often than expected based on the baboons’ relative use of the two sleeping site types (χ2 = 2.48, df = 1, p = 0.12).
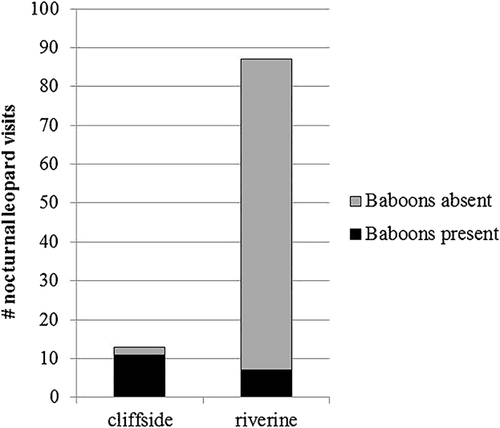
The duration of leopard visits at sleeping sites at night was significantly longer when baboons were present (N = 18, median = 90 min, range = 15–420 min), than when baboons were absent (N = 78, median = 30 min, range = 15–705 min) (UA = 970.5, z = −2.52, p = 0.01, 2-tailed). On an individual level, leopards were consistent with each other in staying at sleeping sites longer when baboons were present (median durations: 60, 75, and 120 min) than when baboons were absent (median durations: 30, 37.5, and 45 min). The duration of leopard visits to baboon-occupied sites did not differ significantly based on site type (cliffside: N = 11, median = 60 min, range = 15–720 min, riverine: N = 7, median = 120 min, range = 15–420 min) (UA = 35.5, z = 0.23, p = 0.82, 2-tailed).
To further examine their apparent lack of preference for certain baboon sleeping sites at night, we compared frequencies of nocturnal versus diurnal visits to cliffside and riverine sleeping sites. If leopards visited baboon sleeping sites with little regard for the presence of baboons, then there should be no significant difference between nocturnal and diurnal visit frequencies. We found that leopards visited baboon sleeping sites less often (N = 77) during the day (06:00–17:45) than at night, which is not unexpected for a largely nocturnally active predator, but that the pattern of diurnal visits to cliffside sites (16; 21%) and riverine sites (61; 79%) was indeed not significantly different from the nocturnal pattern (χ2 = 1.92, df = 1, p = 0.17).
Individual leopards differed in their access to baboon sleeping sites (Figure 1). Adult female CH's home range included no sleeping sites used by our baboon study groups, while adult female KO's home range included two riverine sleeping sites and two cliffside sleeping sites, adult female HA's home range included three riverine sites and two cliffside sites, and subadult male TA's much larger home range included all 11 riverine sites and six cliffside sites. KO never visited riverine sites despite having access to them, and visited cliffside sleeping sites more often when baboons were present (N = 5) than when they were absent (N = 2). HA and TA visited riverine sleeping sites more often than they visited cliffside sites. However, their visits to riverine sleeping sites occurred more often when baboons were absent than when they were present (N = 76 vs. 7, respectively), and they only visited cliffside sleeping sites on nights when baboons were present (N = 6).
3.3 Nocturnal visits by leopards to baboon sleeping sites: Moon phase
One of the adult females (KO) and the subadult male (TA) visited sleeping sites at night slightly more often during the new moon than during other moon phases (KO: 42%, N = 7; TA: 38%, N = 39), whereas adult female HA visited them slightly less often during the new moon (HA: 31%, N = 54). However, these patterns were not significantly different (χ2 = 1.24, df = 4, p = 0.87). Moon phase also did not influence the frequency of visits by leopards to the two types of sleeping site (χ2 = 0.44, df = 2, p = 0.80). The duration of nocturnal leopard visits was not affected by moon phase either when baboons were present (Kruskal-Wallis: H = 0.01, df = 2, p = 0.99), or when baboons were absent (H = 0.76, df = 2, p = 0.68).
3.4 Sleeping site behavior of baboon groups: Site fidelity
The baboon groups spent a mean of 3.0 (±5.3 SD) consecutive nights at the same sleeping site. However, there was considerable intergroup variation in duration of site use (Table 2). While ST Group and MG Group returned to the same sleeping site for 2.1 and 2.4 nights on average, respectively, LI Group and AI Group returned to the same site for a mean of 3.8 and 7.2 consecutive nights, respectively. AI Group had the five longest bouts of consecutive nights spent at any sleeping site (29, 42, 43, 50, and 59 nights), all of which occurred at the Baboon Cliffs sleeping site (Table 3). The number of times groups returned to the same sleeping site (N = 13) or moved to a new site (N = 5) following nights when a collared leopard visited did not differ significantly from nights when they did not visit (χ2 = 0.26, df = 1, p = 0.61). However, we note that not all leopards in the study area were collared and the results could be affected by un-collared leopards if they visited sleeping sites on nights when collared leopards did not visit. We thus compared site fidelity using the subset of 18 visits by collared leopards to baboon-occupied sleeping sites, with the assumption that the likelihood of visits by multiple leopards to the same group on the same night would be small since leopards are solitary hunters, and no two collared leopards ever visited the same group on the same night. We found no evidence that groups were more likely to move to another site after collared leopards visited. In fact, we found the opposite trend. Groups returned to the same sleeping site more often (72% of nights; N = 13) than they switched sleeping sites (28% of nights; N = 5) although the difference was not significantly greater than expected from chance (χ2 = 2.72, df = 1, p = 0.10).
3.5 Sleeping site behavior of baboon groups: Departure and arrival times
There is little variation in sunrise time at MRC as it is near the equator (mean sunrise time: 06:29, range: 06:13–06:44). The baboons’ median departure time from sleeping sites was 07:30 (range: 06:00–11:00, ±53.2 min SD), with little intergroup variation (group medians ranged from 07:00–07:45; ±39.4–59.0 min SD). Only 5% of all departures (60 of 1251) occurred before sunrise. The median departure time from riverine sites was 07:15 (range: 06:30–10:15, ±43.6 min SD), while the median departure time from cliffside sites was 07:30 (range 06:00–11:00, ±55.1 min SD). Similar to overall departures, only 6% (1 of 18) of departures following a collared leopard visit occurred before sunrise. The median departure time following collared leopard visits to riverine sleeping sites (07:45, ±52.6 min SD, N = 7) was 30 min later than on non-visit group-nights (07:15, ±43.5 min SD, N = 256), whereas the median departure time following collared leopard visits to cliffside sites (07:15, ±32.8 min SD, N = 11) was 15 min earlier than on non-visit group-nights (07:30, ±53.3 min SD, N = 977). Baboons departed significantly later from riverine sites than from cliffside sites following leopard visits (UA = 62, z = −2.08, p = 0.04, 2-tailed).
The median time of arrival at sleeping sites by all groups was 18:00 (range 9:45–21:15, ±55.7 min SD), 36 min earlier than mean sunset time (18:36). However, 24% (294) of all arrivals (N = 1251) occurred after sunset (group medians: 17:45–18:00) (Tables 2 and 4). The median arrival time at riverine sites was 18:30 (range: 09:45–19:30, ±55.9 min SD), while the median arrival time at cliffside sites was 18:00 (range: 14:15–21:15, ±55.0 min SD). The median arrival time at sleeping sites for all baboon groups on nights directly following a nocturnal visit from a collared leopard was 18:00, which corresponds exactly to the overall median arrival time and the median arrival time following nights without visits (Table 4). There was no significant difference in arrival times to riverine and cliffside sites following leopard visits (UA = 47, z = −0.72, p = 0.47, 2-tailed). Following the 18 nights when collared leopards visited baboon-occupied sleeping sites, on four occasions (22%) baboons arrived at their next sleeping site after sunset. This is similar to the overall pattern of before and after sunset arrivals (χ2 = 0.02, df = 1, p = 0.89).
| Baboon study groups | |||||
|---|---|---|---|---|---|
| All groups | AI | LI | ST | MG | |
| LV nightsa (N) | 18 | 9 | 4 | 2 | 3 |
| NV nightsb (N) | 1235 | 351 | 161 | 374 | 347 |
| LV departure time (median) | 07:30 | 07:30 | 07:38 | 07:45 | 07:15 |
| NV departure time (median) | 07:30 | 07:45 | 07:15 | 07:45 | 07:00 |
| LV return to site (N) | 12 | 9 | 2 | 1 | 1 |
| NV return to site (N) | 819 | 302 | 118 | 200 | 199 |
| LV move to new site (N) | 6 | 0 | 2 | 1 | 2 |
| NV move to new site (N) | 413 | 49 | 43 | 174 | 147 |
| LV arrival time (median) | 18:00 | 17:45 | 18:15 | 18:45 | 17:45 |
| NV arrival time (median) | 18:00 | 17:45 | 18:30 | 18:30 | 17:30 |
- a Leopard visit (LV) group-nights include those in which a collared leopard came within 160 m of an occupied baboon sleeping site.
- b Non-visit (NV) group-nights include those in which no collared leopard came within 160 m of an occupied baboon sleeping site.
3.6 Sleeping site behavior of baboon groups: Co-sleeping
Nocturnal hunting of baboons by leopards and the benefits of dilution as an antipredator strategy might favor separate groups sleeping at the same site on the same night. Co-sleeping occurred on 46 of 1253 group-nights (4%), always with only two groups. All but two of these nights involved ST Group, the smallest of the four groups. ST Group was primarily responsible for joining larger groups. It joined other groups that had arrived earlier to the sleeping site on 28 of 44 nights whereas it was joined by a larger group on only four nights (χ2 = 16.54, df = 1, p < 0.001). On 12 nights ST Group arrived at the same time as the larger group. All but two nights of sharing occurred on cliffside sites.
3.7 Sleeping site behavior of baboon groups: Vocalizations
Baboon vocalizations at the Hippo Pool riverine sleeping site were common at night, and mainly consisted of barks, wahoos, screams, and squeals. Barks and wahoos were recorded on 32 of 35 (91%) nights during 1–10 hr on a given night. On at least 10 (31%) of the nights during which they vocalized, leopard presence at the sleeping site was documented via GPS data, camera traps, or leopard vocalizations. We note that baboon presence at the Hippo Pool sleeping site was greater than revealed by the GPS data (Table 3) because one group (LI) that used the site lost both collared females mid-way through the study. After those females died, we confirmed the presence of LI Group at that sleeping site on 21 additional nights through direct observation as the group departed the following morning, when we were able to identify individually recognizable females (we did not use these data in our analyses because we did not have data on where the group slept on the other nights).
4 DISCUSSION
Remote monitoring technology allowed us to overcome previous obstacles to studying predator-prey interactions involving primates, particularly at night. However, non-experimental, field-based behavioral research is inherently subject to uncertainty. For instance, our study subjects were fewer than expected because one of the collared leopards did not visit sleeping sites used by baboon study groups and the only collared female baboons to die were both from the same group, eventualities that we could not know or predict ahead of time. Because our study is the first of its kind, it is impossible to know if our study animals and study site are representative. We encourage others to conduct similar studies in other ecosystems to investigate the potential for variation in leopard-baboon interactions at baboon sleeping sites.
Investigating leopard and baboon movements recorded simultaneously and remotely via GPS collars allowed us to examine patterns of leopard visits to baboon sleeping sites and assess the use of sleeping sites by baboons relative to those visits. Baboons are more vulnerable at night than during the day partly because they are less likely to counter-attack at night (Busse, 1980; Cowlishaw, 1994; Isbell et al., 2018). Our observations of baboons chasing a leopard away from a riverine sleeping site during diurnal hours (07:00), and camera trap photos showing a leopard leaving the same site with a baboon kill during nocturnal hours (05:14) without a chase (Isbell et al., 2018) suggest that baboons are hampered at night, likely because their night vision is poorer than that of leopards.
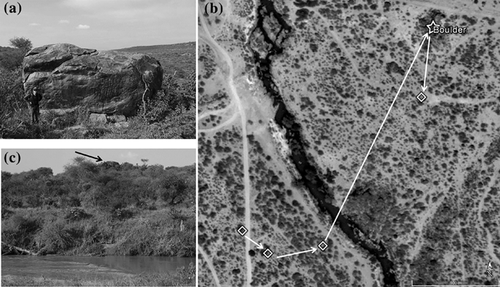
It may seem paradoxical that sleeping sites, which are thought to be places of refuge, are where baboons are most vulnerable to leopard predation. The adaptive explanation is that they would be even more vulnerable if they did not go to tall trees or cliff faces at night. Indeed, the one predation event on a collared baboon by a collared leopard occurred on a small boulder (Isbell et al., 2018) located 180 m from the river (Figure 3). Based on the locations of her collared groupmate, for 6 days the victim was separated from her group (Isbell et al., 2018), traveling only short distances, and sleeping at riverine sites until the night before she was killed. We surmise that she was ill and unable to move the distance required to keep up with the group or make it to tall trees on the night she went to the boulder. This was the only time the boulder was used as a sleeping site, and it was ineffectual.
4.1 Leopard behavior at baboon sleeping sites
Leopards elsewhere have been reported to make more kills during phases of the moon with less moonlight (Martins & Harris, 2013), but we found little effect of moon phase on leopards’ nocturnal visits to baboon sleeping sites. This suggests that leopards approached sleeping sites even when moonlight could have made them more visible to baboons, and it is consistent with other evidence, for example, nocturnal predation events, that leopards are emboldened to hunt baboons at night.
Certain cliffside sleeping sites (e.g., Baboon Cliffs) constituted reliable locations at which to find baboons at night, and so for Mpala leopards, those sites can be considered prey hotspots (Emsens et al., 2014). Our earlier research showed that leopards sometimes moved directly to such sleeping sites when baboons were there (Isbell et al., 2018). Nonetheless, in general, we found that collared leopards did not visit cliffside sites more often than riverine sites when baboons were present at those sites, after taking into account the relative use of such sites by baboons. It is worth considering the possibility that leopards visit cliffside baboon sleeping sites unpredictably, making it difficult for baboons to gain more information about the risk of attack (Roth & Lima, 2007). This would not apply to leopard movements to baboon-occupied sleeping sites in the riverine habitat, however, because leopards regularly travel along the river at night (Isbell et al., 2018; Van Cleave et al., 2018). Their visits to baboon-occupied riverine sleeping sites were likely driven by other factors that lead leopards along the river, such as the extent of cover and the presence of other prey. Hunting baboons at night along the river was likely opportunistic and dependent on the leopards’ motivation to hunt.
The motivation to hunt is often overlooked because it is typically assumed that predators of primates do not turn down opportunities to hunt them. Nonetheless, it is important to consider as it can explain some observed variation in predation risk. For instance, despite being within hunting proximity to baboons both day and night, leopards appear to be more motivated to hunt them at night than during the day (Isbell et al., 2018). Indeed, when leopards encountered baboons at sleeping sites at night, they remained nearby three times longer than at unoccupied sites, regardless of the type of sleeping site.
Similarly, motivation affects leopard hunting behavior toward vervet monkeys (Chlorocebus pygerythrus). Our earlier research revealed that although leopards at night often directly approached vervet groups, this was more a reflection of the physical constraint of moving along the river than an interest in hunting them since leopards killed vervets only during the day (Isbell et al., 2018), and moved away quickly from alarm-calling vervets at night (Isbell & Bidner, 2016). Although leopards frequently approached vervets (Isbell et al., 2018) and only occasionally approached baboons as they moved along the river at night (this study), they appeared to be more motivated to hunt baboons, perhaps because baboons are within the preferred body mass range of leopards, or because they were easier to see or reach in trees. On at least one occasion when a leopard encountered both baboons and vervets at night at a riverine sleeping site, it chose to hunt and kill a baboon (Figure 7 in Isbell et al., 2018). During that night, we recorded many baboon vocalizations but no vervet vocalizations, as if vervets were attempting to avoid detection.
In addition, leopards at Mpala had access to a wide variety of non-primate prey. In 175 24-hr diel periods, three leopards at our study site were documented killing or scavenging mammals ranging in size from African hares (Lepus microtis) to impala (Aepyceros melampus) 81 times (Wilmers, Isbell, Suraci, and Williams, 2017), or approximately once every other day. The time since the last kill and the availability of prey both factor heavily into leopards’ motivation to hunt (Wilmers et al., 2017).
4.2 Baboon behavior at sleeping sites: Preference for cliffside sites and sleeping site re-use
Although most riverine sleeping sites included trees with vines that might facilitate hiding or hamper leopard movement, baboons slept at cliffside sites more often than they slept at riverine sites. We suggest that they prefer cliffside sites because leopards visit riverine sites more frequently and trees provide less effective protection than cliff faces. Leopards are easily able to climb trees but are less able to gain purchase on the steep rocky faces of kopjes (Hamilton, 1982). Previous research has also demonstrated that leopards prioritize the ease in capturing prey over prey abundance in selecting hunting locations (Balme, Hunter, & Slowtow, 2007).
Three of the four baboon groups used cliffside sites as their main sleeping site and for consecutive bouts of up to 59 nights. Repeated use of a few sleeping sites also suggests that baboons perceive such sites to be safer. It has been hypothesized that baboon groups may reduce detection by predators by frequently switching sleeping sites (Altmann & Altmann, 1970; Hamilton, 1982), but we found little evidence to support this. An analysis of sleeping site use by yellow baboons (P. cynocephalus) in Amboseli National Park, Kenya, suggested that despite relatively frequent shifts in sleeping sites by individual groups, this behavior was unlikely to reduce detection by predators because preferred sleeping sites were so often in use by one or another group (Markham et al., 2015). Only two of our study groups (ST and MG) changed sleeping sites as frequently as Amboseli yellow baboon groups did (Markham et al., 2015).
In addition to regularly re-using sleeping sites, the Mpala baboons’ occasional sharing of sleeping sites and their frequent nocturnal vocalizations, at least at one sleeping site along the river, suggest that minimizing detection by predators was not a high priority. As Markham and colleagues (2015) suggested, it may be difficult for a large-bodied, highly gregarious animal to reduce detection by predators as an antipredator strategy.
4.3 Adjusting sleeping site departure and arrival times
Baboon groups regularly departed from both types of sleeping site after sunrise, and by waiting, they would have improved their chances of detecting nearby leopards. Baboons were less consistent in arriving at sleeping sites prior to sunset. Due to decreased visibility after dusk, baboons could have been vulnerable to ambush by leopards during their not infrequent late arrivals. However, the behavior of our collared leopards indicates that they adopted an active nocturnal hunting strategy over one of ambushing baboons at sleeping sites; indeed, collared leopards rarely visited sleeping sites in use by baboons in the two hours before dusk (four occasions) or after dawn (five occasions) during presumably ideal ambush conditions.
We did not find that baboons adjusted their behavior immediately following nocturnal visits by collared leopards. They neither extended their departure time from sleeping sites on mornings following such visits nor arrived earlier at sleeping sites the next evening. In Filoha, Ethiopia, hamadryas baboons (P. hamadryas) departed from cliffside sleeping sites later following nights when lions and/or hyenas vocalized nearby (Schreier & Swedell, 2012). It is possible that baboons in our study did not leave sleeping sites later in the morning following nocturnal visits by collared leopards because they were not always aware of leopard presence. For instance, our acoustic recordings and camera trap photos (see Isbell & Bidner, 2016) showed that leopards vocalized on only one of 47 nights when they were caught on camera at the Hippo Pool riverine sleeping site. Their relative silence might be countered to some degree, however, at least in riverine areas, by vervet vocalizations. When vervets detect leopards they give a distinctive alarm call (Seyfarth, Cheney, & Marler, 1980). At the Hippo Pool sleeping site, vervets gave leopard alarm calls on 32% of nights (Isbell & Bidner, 2016). Thus, by “eavesdropping” on vervets, baboons may have been alerted to leopards more frequently at riverine sleeping sites than at cliffside sites because vervets typically slept along the river and did not sleep at cliffside sites. Indeed, baboons departed from riverine but not cliffside sites later following collared leopard visits. To our knowledge, eavesdropping on heterospecific alarm calls has not been reported for baboons yet, but since it is common among animals (Magrath, Haff, Fallow, & Radford, 2015), we expect baboons to take advantage of vervet alarm calls. We also note that when we observed baboons chasing a leopard away from the Hippo Pool site one morning (Isbell et al., 2018), the baboons initially passed by the thick bush where the leopard was hidden, and only approached the area again after vervets began giving leopard alarm calls from the trees above.
4.4 Sleeping site co-sharing
In Amboseli, baboon groups do not share sleeping sites on the same night, and the most dominant group had greater access to higher quality sleeping sites than the most subordinate group, suggesting that groups compete for sleeping sites (Markham et al., 2015). In our study, sharing occurred on 4% of all baboon-nights, and the pattern of sharing suggests that sleeping sites were not always sources of competition. Although we do not know the rank order of intergroup dominance, assuming larger groups are better competitors than smaller groups, if sleeping sites were resources worth competing over, the smallest group should have been excluded from sleeping sites already occupied by larger groups. In fact, the smallest group was most often responsible for joining other groups at sleeping sites. By sharing a sleeping site, both groups may gain an antipredator benefit by increasing the numbers of conspecifics in proximity at night. With their poor nighttime vision, the benefit to having more baboons present at a sleeping site is more likely to be dilution (Foster & Treherne, 1981) than earlier predator detection.
In summary, our findings suggest that leopards at our study site hunt baboons at their sleeping sites at night mainly opportunistically in that finding an occupied sleeping site piques their interest and may prompt hunting—reflected in the longer times spent at occupied than vacant sites. Baboons appear to be sensitive to their heightened nocturnal vulnerability and mitigate it by preferentially choosing cliffside sleeping sites, regularly departing from sites after sunrise, occasionally sleeping with other groups, and adjusting departure times at riverine sleeping sites following leopard visits. They do not, however, change sleeping sites or adjust arrival times after a leopard visits, or attempt to avoid detection by continually being quiet at night. Their frequent nocturnal vocalizations, at least at one riverine sleeping site, also indicate they are often awake at night. Indeed, data from accelerometers revealed that our collared baboons were active about 15% of the night (Isbell, Bidner, Crofoot, Matsumoto-Oda, & Farine, 2017), and it is possible that groups were collectively alert to potential risks throughout the night. In captivity baboons were found to sleep less deeply than orangutans (Samson & Shumaker, 2015), which could indicate selection for nocturnal alertness to potential risks in baboons, decreased selection for risk response in orangutans (e.g., Lameira et al., 2013), or both. Further research is needed to determine the extent to which baboons are alert to nocturnal risks, and if/how nocturnal baboon activity and vocalizations at sleeping sites affect the dynamics of predator-prey interactions.
Our study has revealed basic aspects of predator-prey dynamics between leopards and olive baboons using GPS monitoring technology. Such remote technology also has great potential to answer other more complex questions about predator-prey interactions within mammalian communities and their ecological implications. For instance, if more frequent activity by leopards along the river is negatively associated with the use of riverine habitat by baboon groups at night, it is possible that this constitutes a behaviorally mediated trophic cascade (e.g., Ford et al., 2014; Ford and Goheen, 2015; Schmitz, Beckerman, & O'Brien, 1997) in which changes in riverine vegetation and nutrient cycling may be indirectly driven by predation risk. Baboon groups used riverine sleeping sites more frequently in 2012 (L.R. Bidner, unpublished data), so longer-term data on sleeping site use and effects of baboons on riverine vegetation are needed, in addition to data on leopard activity, to investigate the trophic effects of this predator-prey interaction. Remote GPS monitoring has the potential to greatly increase our understanding of non-lethal effects of predation involving flexible, difficult-to-study predators and their behaviorally and ecologically flexible prey.
ACKNOWLEDGMENTS
We thank the National Commission for Science, Technology, and Innovation of Kenya (P/15/5820/4650) for permission to conduct research in Kenya and the Kenya Wildlife Service for local affiliation. We are grateful to Margaret Kinnaird, Lawrence Frank, and Truman Young for logistical support, Mathew Mutinda and George Omondi for veterinary assistance, Steven Ekwanga and Dairen Simpson for leopard trapping, and Matt Snider, Eric Van Cleave, Masaki Iwata, Andrea Dominicos Surmat, Martin Gichuru, Francis Lotukoi Emojo, and especially Wilson Longor for field assistance. We also thank Dan Rubenstein for loaning us camera traps including the one that captured leopard and baboon interactions at Hippo Pool. We gratefully acknowledge funding from the Wenner-Gren Foundation (grant 8386) to LRB, the National Science Foundation (BCS 99-03949 and BCS 1266389), L.S.B. Leakey Foundation, and UC Davis Faculty Research Grants to LAI, and JSPS KEKENHI (grant no. 23405016) to AMO. We also thank Marilyn Norconk, Lynne Miller, and one anonymous reviewer for their thorough review of and comments on previous versions of this paper.




