High reproductive effort is associated with decreasing mortality late in life in captive ruffed lemurs
Abstract
Evolutionary theories of senescence predict that a high allocation to reproduction during early life should have long-term deleterious consequences on future reproduction or survival because individuals have to face an energy allocation trade-off between reproductive effort and the maintenance of body condition. Using a high-quality dataset from 1,721 red ruffed lemurs (RRL, Varecia rubra) and 3,637 black and white ruffed lemurs (BWRM, V. variegata) living in captivity, we tested the existence of a trade-off between reproductive effort and late-life survival after accounting for possible confounding effects of natal environmental conditions. We report clear evidence of actuarial senescence (i.e., the decline of annual survival with increasing age) in both sexes and for both species of ruffed lemurs. RRL had a lower baseline mortality and senesced faster than BWRL, resulting in similar distributions of longevities for both species. No between-sex difference was observed in any species. Lastly, a higher reproductive effort was positively associated with an increase of survival late in life, and thereby an increased longevity. These findings indicate that individual quality rather than trade-off drives the association between reproductive success and survival pattern among individual lemurs of both species in the protected environment provided by zoos. Lemurs are among the world's highest conservation priorities and better understanding factors influencing their longevity and actuarial senescence patterns should improve their conservation.
1 INTRODUCTION
Numerous species are currently threatened by extinction in their natural habitat due to anthropogenic activities (IUCN, 2012). Among them, lemurs are considered as a world's highest conservation priorities (Brummitt & Lughadha, 2003; Sechrest et al., 2002). Nowadays, around 90% of lemur species are threatened by extinction in the wild (Schwitzer et al., 2013), with 17 out of ca. 52 species of lemurs considered as endangered or critically endangered (IUCN, 2012). Lemurs are endemic from Madagascar and their main threats are the degradation of the natural habitat with a decrease of as much as 80–90% of the original forest cover (Green & Sussman, 1990) and poaching by local populations (Borgerson, 2015), even within protected areas (Goodman & Raselimanana, 2003). In this context, maintaining self-sustaining captive populations can be seen as a safeguard against extinction with the aim to use individuals born in zoos to reinforce or re-establish wild populations. For instance, the number of lion golden tamarin (Leonthopitecus rosalia) in the wild has more than doubled between the late 1970s and 1994 after the reintroduction of 71 captive-bred individuals in the 1980s (Beck et al., 1991; Kleiman et al., 1986). Reintroduction of captive-bred individuals of lemur species has also been done successfully in Madagascar (Britt, Welch, & Katz, 2004). Among lemurs, the two species belonging to the genus Varecia (ruffed lemurs) are particularly threatened in the wild because their highly specialized frugivorous diet (Britt et al., 2004; Vasey, 2003) makes them especially vulnerable to decreased availability of feeding sites caused by human-driven habitat loss. Being the largest members of Lemuridae (ca. 3.5 kg), ruffed lemurs are also threatened by hunters for their meat (Borgerson, 2015).
Actuarial senescence corresponds to the progressive decline of annual survival with increasing age (Monaghan, Charmantier, Nussey, & Ricklefs, 2008; Rose, 1991). Actuarial senescence is an almost ubiquitous process in age-structured populations (Nussey, Froy, Lemaître, Gaillard, & Austad, 2013) but displays highly variable patterns across the tree of life (Jones et al., 2014). Actuarial senescence has been reported in captive (Lemaître, Gaillard, Bingaman Lackey, Clauss, & Müller, 2013; Ricklefs, 2000, 2010; Tidière et al., 2014, 2015) as well as in wild (Jones et al., 2008, 2014; Nussey et al., 2013) populations, but is often delayed in zoo, at least for mammals, thanks to protected environments offered by zoos (Lemaître, Gaillard, Bingaman Lackey, Clauss, & Müller, 2013; Tidière et al., 2016). A trade-off between energy allocation in growth and/or reproduction early in life, and somatic maintenance is frequently proposed to explain the existence of actuarial senescence (Kirkwood & Rose, 1991). Indeed, any increase of energy allocation to early-life fitness-related traits should lead to a decrease of energy available to somatic maintenance (e.g., DNA repair mechanisms), resulting in earlier and/or faster actuarial senescence, and thereby leading to a shorter longevity.
To date, numerous empirical studies of vertebrate populations have provided support for the existence of such trade-offs between early- and late-life performance (Lemaître et al., 2015). Early-life performance can be measured using different metrics such as body growth rate, age at first reproduction (AFR), or reproductive effort during the prime-age stage (e.g., total number of offspring produced or successfully weaned, mean litter size). For late life, performance can be measured using complementary metrics of survival (e.g., longevity, baseline mortality) and actuarial senescence such as the age at the onset of senescence, which measures the timing of senescence, and the rate of senescence, which measures the strength of senescence (Tidière et al., 2015). As pointed out by recent studies (Berger et al., 2016; Gamelon et al., 2014; Tidière et al., 2015), it is crucial to consider both the onset and the rate of senescence to assess reliably the pattern of actuarial senescence in a given population.
In this study, we investigated potential long-term costs of reproduction in both sexes of captive populations of red ruffed lemurs (RRL, Varecia rubra) and black and white ruffed lemurs (BWRL, V. variegata). This topic cannot been addressed in the wild because declining populations of red ruffed lemurs and black and white ruffed lemurs (IUCN, 2012) are extremely difficult to study in their natural environment due to their fragmented distribution (Irwin, Johnson, & Wright, 2005). Very little is known on the potential early-late-life trade-offs in non-human primates (but see Blomquist, 2009). Long-term reproductive costs are likely to be particularly pronounced in ruffed lemurs, especially in females, because of their high level of maternal care. First, both species of ruffed lemurs are currently the only extant species of the genus Varecia and display life histories unusual for Primates. Despite being large (3.5–3.6 kg on average for both sexes, Terranova & Coffman, 1997), ruffed lemurs have both relatively short gestation length (102 days) and high mean litter size (1.89 for RRL and 2.22 for BWRL) (Whipple, 2014, 2016) compared to other lemurs (120–136 days, Bogart, Cooper, & Benirschke, 1977; Boskoff, 1977; Brockman, Willis, & Karesh, 1987; Foerg, 1982; Rasmussen, 1985; Shideler, Lindburg, & Lasley, 1983; and 1.0–1.6, Rasmussen, 1985, respectively). Moreover, ruffed lemurs show one of the fastest embryo growth rates relative to maternal body mass among Strepsirrhini primates (Young, Richard, & Aiello, 1990). Finally, female ruffed lemurs produce the richest milk of lemurs (Baden, Wright, Louis, & Bradley, 2013; Vasey, 2007). This brief life history account indicates that ruffed lemurs allocate a lot to both pre- and post-natal care compared to other lemurs (Pereira et al., 1987; Young et al., 1990). Second, in the wild, ruffed lemur populations live in the costal rainforest of eastern Madagascar in multi-male and multi-female communities including 18–30 individuals in a fusion-fission dynamic with little or no intra-sexual competition for dominance (Morland, 1990b; Vasey, 2003, 2007). There is some evidence that only females participate to communal home range defense against females of other groups (Morland, 1991a; Vasey, 2006). Moreover, at least in captivity, females are consistently observed to be dominant over males (Kaufman, 1991; Meyer, Gallo, & Schultz, 1999), likely through the access to food resources. In the wild, the female dominance is more difficult to assess due to inter-group variation in dominance patterns (Overdorff, Erhart, & Mutschler, 2005). However, female feeding priority has been observed in wild populations of ruffed lemurs and this female group dominance has been interpreted as a behavioral tactic that allows females to cope with unusually high reproductive energy expenditures (Young et al., 1990). Lastly, Varecia reproduce annually, synchronously, and are highly seasonal breeders (Baden et al., 2013; Vasey, 2007). Their mating system is polygynandrous (or promiscuous), meaning that one male mates with several females (from the same group/community or from another group) and one female mates with different males (from the same group/community or from another group), and there is no sexual size dimorphism (Vasey, 2007). During the mating season, aggressive behavior increases among individuals of the same sex, but also between females and males (Foerg, 1982; Morland, 1993). Aggressive behavior by females is often directed toward the male who is attempting to mate with them, by hitting and biting the male (Vasey, 2007). Deaths due to injuries following mating are sometimes observed in captivity (Whipple, 2014, 2016). Ruffed lemurs are highly social animals that rear communally young with alloparental care (Baden et al., 2013; Vasey, 2007). Offspring are altricial and parents often hide them in nests. Only females feed young but infants are guarded by all males and females of the community, and females doing communal nesting have a higher reproductive success than other females (Baden et al., 2013). Therefore, infant care-providers are not necessarily genetic (mating) partners, and alloparenting has been reported to occur in captivity (Pereira, Klepper, & Simons, 1987) as well as in the wild (Morland, 1990a).
As ruffed lemurs allocate a lot to maternal care, their reproduction is energetically costly. We thus expect individuals producing many offspring to have less energy available for somatic maintenance and to show then an earlier and/or stronger senescence, associated to a later age at death, than individuals reproducing actively early in life. Thanks to a high-quality dataset provided by the International Ruffed Lemur Studbook (Whipple, 2014, 2016), which compiles individual data of all ruffed lemurs living in captivity (from 195 and 277 institutions for RRL and BWRL, respectively), we tested for a trade-off between reproductive cumulative effort and late-life performance in RRL and BWRL. The studbook includes individual information on sex, dates of birth and death, and parent identification numbers, for an historical population of 1,954 RRL and 4,169 BWRL.
In a first step, we determined the general sex- and species-specific patterns of actuarial senescence. We estimated the age at the onset of senescence, the rate of senescence, the baseline mortality (i.e., minimum mortality at the age when actuarial senescence starts), the longevity (calculated as the age at which 90% of individuals from the initial population were dead, which provides the most relevant metric of longevity at the population level, Moorad, Promislow, Flesness, & Miller, 2012) and the maximum longevity (observed in the historical populations). While no difference should occur between these closely related species sharing most of ecological and physiological traits (Whipple, 2014, 2016), between-sex differences of actuarial senescence and longevity are generally expected in mammals. In non-monogamous mammals, males often senesce earlier, faster, and have a shorter life than females, both in captive and free-ranging populations. For instance, in ruminant species, several studies reported an earlier and faster actuarial senescence in males than in females (Catchpole, Fan, Morgan, Clutton-Brock, & Coulson, 2004; Loison, Festa-Bianchet, Gaillard, Jorgenson, & Jullien, 1999; Müller, Gaillard, Bingaman Lackey, Hatt, & Clauss, 2010; Toïgo et al., 2007), possibly due to the higher male allocation to costly traits and agonistic behaviors during the mating period (e.g., growing larger than females, Ricklefs & Scheuerlein, 2001; riskier behaviors through dispersal, and territorial defense, Tecot, Gerber, King, Verdolin, & Wright, 2013] than females (Bro-Jørgensen, 2012; Müller et al., 2011; Tidière et al., 2015). A shorter longevity and stronger senescence in males have also been reported in wild populations of primates (Bronikowski et al., 2011). However, in both ruffed lemur species, there is no sexual size dimorphism and the level of intra-sexual competition to mate is low, indicating little or no sex-biased allocation in intra-sexual competition to mate. Moreover, while energy allocation in embryo growth, parturition, and lactation is only provided by females, males are subject to female aggressive behaviors that potentially lead these males to injuries, and cost of raising offspring are shared by all individuals of the community. Then, we expect no between-sex differences in actuarial senescence of both Varecia species. In support, in a polygynandrous primate without male-male competition during mating (Northern muriquis, Brachyteles hypoxanthus), Bronikowski et al., (2011) did not report any between-sex difference of actuarial senescence.
In a second step, we tested the existence of a trade-off between reproductive effort and late-life performance in captive individuals of ruffed lemurs after accounting for possible confounding effects of natal environmental conditions. We measured the reproductive effort as a reproductive cumulative success using three different metrics, corresponding to the cumulative number of offspring produced (hereafter named RCST for reproductive cumulative success in total), the cumulative number of offspring successfully weaned (hereafter named RCSW) and the cumulative number of litters (hereafter named RCSL). RCST and RCSW correspond to the physiological costs associated with gestation, parturition, lactation, and raising offspring while RCSL corresponds to the proportion of lifetime during which individuals have allocated energy to reproduction. According to current life-history theories of aging (Kirkwood, 2017; Kirkwood & Rose, 1991; Lemaître et al., 2015; Vaupel, 2010), we expected that the increase of reproductive effort starting in early-life should negatively influence late-life survival, resulting in a stronger (i.e., earlier and/or faster) actuarial senescence and a shorter longevity. Moreover, due to the high amount of energy allocated by females to milk production (Baden et al., 2013; Vasey, 2007) and because gestation costs are lower than lactation costs in mammals (Clutton-Brock, Albon, & Guinness, 1989), we expected stronger costs induced by RCSW compared to RCST in terms of actuarial senescence in females.
To summarize, the goals of our study are (i) to assess the survival and actuarial senescence patterns for males and females of two closely related lemur species living in captivity with no between-sex difference expected; and (ii) to test the existence of a trade-off between cumulative reproduction and late-life survival in captive individuals of ruffed lemurs.
2 METHODS
2.1 Study populations
Data were obtained from the published International Studbook for Ruffed Lemurs (Whipple, 2014, 2016). This studbook compiled data for the majority of Red Ruffed Lemur (RRL, V. rubra) and Black and White Ruffed Lemur (BWRL, V. variegata) living in captivity in the world (e.g., zoos, primate centers) from 1959 to 31st December 2015. The studbook provides, for each individual, sex (male, female, unknown), date and location at birth, date and location of each transfer, and date and location at death, if the individual died before 1st January 2016. For some individuals, the monitoring was lost after a transfer. For them, the date is indicated in the studbook as “lost to follow up” and their capture-histories were right-censured for analyses. A total of 1,954 individuals of V. rubra (RRL) and 4,169 individuals of V. variegata (BWRL) are identified in the studbook. All individuals for which the sex and/or the date of birth were unknown have been removed from the dataset. We then obtained a final dataset of 1,721 (756 females and 965 males) and 3,637 (1,589 females and 2,048 males) individuals from V. rubra and V. variegata, respectively. For the sake of simplicity, males and females of RRL and BWRL have been analyzed as four distinct datasets.
The research reported in that work complied with protocols approved by the appropriate Institutional Animal Care Committee. The research adhered to the legal requirements of the country in which the research was conducted; and the research adhered to the American Society of Primatologists (ASP) Principles for the Ethical Treatment of Primates.
2.2 Data analyses
2.2.1 General pattern of actuarial senescence
We first determined the general actuarial senescence pattern for each sex separately for both species. For each sex of both species, we built from studbook data the capture history of each individual, coded as “1” when the individual was observed alive and as “0” when it was dead in a given year. For individuals with an interrupted monitoring, we censured the capture history when the individual was lost. For all individuals still alive in 2016, we censured their capture history at the 1st of January, 2016. These capture histories were then loaded into the CMR (capture-mark-recapture) software E-SURGE (Choquet, Rouan, & Pradel, 2009) to get reliable age-specific estimates (Lebreton, Burnham, Clobert, & Anderson, 1992), knowing that detection probabilities were equal to 1 in captivity.
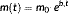
with m(t) corresponding to the mortality rate at each time t, m0 is the baseline mortality and b is the Gompertz rate of mortality increase. This function is the most commonly used to study human survival or survival of mammals and birds in captive conditions (Ricklefs & Scheuerlein, 2002) and has been shown to provide a reliable description of age-specific survival in primates (Bronikowski et al., 2011; Larson, Colchero, Jones, Williams, & Fernandez-Duque, 2016). Gompertz model is also particularly relevant in a senescence context because its parameters m0 and b have a direct biological interpretation. Then, the baseline mortality measures the minimum mortality rate before senescence starts, and the Gompertz rate (called “rate of senescence” hereafter) measures the average exponential rate of mortality increase with age (Table 1). On a logit scale, age variation in survival is constrained to be linear from the age at the onset of senescence onwards (Gaillard, Viallefont, Loison, & Festa-Bianchet, 2004). To select the best model, we used a model selection procedure based on the Akaike Information Criterion (Burnham & Anderson, 2002). We retained the model with the lowest AIC (Table S1). For the four datasets, at least one of the Gompertz model consistently provided a better fit than either the constant or the two-age class model.
| Red ruffed lemur | Black and white ruffed lemur | |||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Number of individuals | 756 | 965 | 1589 | 2048 |
| Longevity 90% (years) | 28 | 29 | 28 | 28 |
| Maximum longevity (years) | 35 | 34 | 35 | 40 |
| Baseline mortality (95%CI) | 0.017 (0.013;0.021) | 0.022 (0.018;0.026) | 0.031 (0.028;0.036) | 0.036 (0.032;0.040) |
| Onset of senescence (years) | 5 | 9 | 4 | 4 |
| Rate of senescence (95%CI) | −1.909 (−2.221;−1.597) | −1.901 (−2.226;−1.577) | −1.151 (−1.353;−0.950) | −0.929 (−1.103;−0.754) |
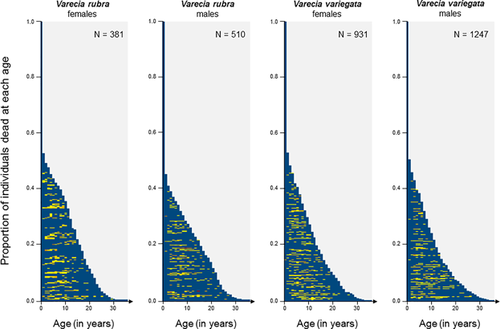
Finally, from the full age-dependent model, we obtained age-specific survival estimates that allowed calculating the proportion of individuals alive at each age (i.e., lx series). We then estimated the longevity (age at which 90% of individuals from the initial population were dead) for each sex in each species. Longevity is not a direct measure of actuarial senescence because it is not age-dependent, but provides information on the survival pattern. We also recovered the maximum longevity observed in the historical population for males and females of RRL and BWRL. We finally obtained for males and females of RRL and BWRL the longevity, the maximum longevity, the baseline mortality, and the onset and the rate of actuarial senescence (Table 1). We then compared sexes and species using standard Wald tests.
2.2.2 Effect of reproductive effort on age at death and rate of senescence
We determined whether cumulative reproductive effort affects survival late in life, and then patterns of actuarial senescence. To assess the influence of energy allocation to reproduction on survival late in life, we used three different metrics (i.e., RCST, RCSW, and RCSL). We tested whether one or all of these metrics affected actuarial senescence and then longevity. We only included individuals that lived beyond the age of sexual maturity (i.e., 1.8 years, Foerg, 1982; Whipple, 2014, 2016) and we removed individuals without any reproductive event during their lifetime from the dataset (but note that the analysis including these latter individuals did not influence the results, see Table S2 and Figure S1). We then analyzed separately 128 female RRL, 104 male RRL, 269 female BWRL, and 239 male BWRL. We performed a non-parametric survival analyses using the “eha” package (Broström, 2016) with R version 2.14.0 (R Development Core Team, 2011). Using a proportional hazard regression, we fitted a Cox model (beginning at the age of sexual maturity) for males and females and for both RRL and BWRL. We then tested the effect of each metric of reproductive effort separately. We controlled for potential effect of individual heterogeneity by including individual covariates in the model (thus accounting for measured heterogeneity, see Plard et al., 2015). We included the age at first reproduction (AFR) because the age at death cannot occur prior to the first observed reproductive event. The dates of birth of captive ruffed lemurs ranged from 1959 to 2015, which encompassed periods with different management rules. Thus, about 30 years ago, the Association of Zoos and Aquariums started to emphasize and share husbandry standards to improve captive conditions for animals. To control for that, we included the decade of birth as a fixed factor in the model (i.e., decade 0: 1959–1965; decade 1:1966–1975; decade 2: 1976–1985; decade 3: 1986–1995; decade 4: 1996–2005; decade 5: 2006–2015) and expected a positive effect to occur on survival late in life. Finally, because ruffed lemurs were born in about 200 different zoos, which might differ in the quality of animal care, we included the zoo of birth as a random effect. The selection of variables to retain was done using a step-by-step selection procedure by removing at each step the variable with the lowest Wald statistic until all variables remaining in the model had a statistically significant effect. Finally, the random effect of zoo of birth was kept in the model when the variance of this effect was larger than 0.1.
3 RESULTS
3.1 Pattern of actuarial senescence in both sexes of the two Varecia species
For each sub-population, at least one of the Gompertz models consistently had a lower AIC than the constant, the two-age class or the full age-dependent models (Table S1). This outcome demonstrates the firm existence of actuarial senescence in both sexes of both ruffed lemur species living in captivity (Figure 1). Wald tests revealed that RRL of both sexes had lower baseline mortality and similar onset of senescence, but displayed faster rates of senescence than BWRL (Table 1). These age-specific survival patterns led to a similar longevity of about 28–29 years in females and males of both species (Table 1). Moreover, we did not detect any between-sex difference in terms of survival or actuarial senescence, although females of both ruffed lemurs species tended to have lower baseline mortality and faster rates of senescence than males, although this between-sex difference was not statistically significant (Table 1).
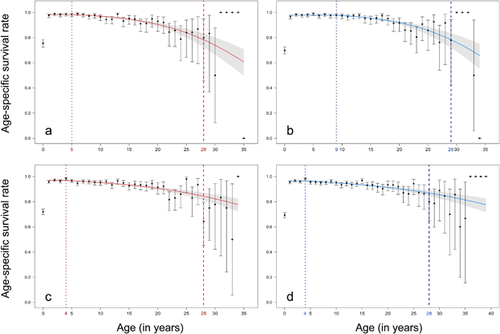
3.2 Effect of reproductive effort on late in life survival and longevity
The random effect of zoo of birth influenced the age-specific survival pattern of females of both ruffed lemur species, but not that of males (Table 2). Then, the effect of the zoo of birth was kept in the model for females but not for males in each species. However, any effect of year of birth was highlighted. Moreover, for individuals that reproduced at least once during their lifetime, the mean AFR was 5.4 (95%CI: [5.0; 5.8]) and 6.2 years of age (95%CI: [5.6; 6.9]) for females and males of RRL, respectively. Females and males of BWRL reproduced for the first time at 5.0 (95%CI: [4.7; 5.3]) and 5.8 (95%CI: [5.4; 6.2]) years of age, respectively. Then, for both species, females tended to reproduce for the first time earlier than males, and whatever the sex BWRL tended to have a first reproduction slightly earlier than RRL. Finally, we could observe graphically that reproduction occurred all along the lifespan for females and males in both species, although with a lower number of offspring produced beyond 20 years of age, which suggests the existence of a reproductive senescence in this species (Figure 2).
| Species | Sex | Variables | β | 95%CI | Variance of random effect (Zoo of birth) |
|---|---|---|---|---|---|
| Red ruffed lemur Varecia rubra | Females (N = 128) Males (N = 104) | RCST | 0.952 | 0.923;0.981 | 0.12 |
| RCSW | 0.945 | 0.910;0.981 | 0.10 | ||
| RCSL | 0.876 | 0.812;0.946 | 0.10 | ||
| RCST | 0.957 | 0.927;0.988 | <0.001 | ||
| AFR | 0.925 | 0.882;0.972 | |||
| RCSW | 0.941 | 0.902;0.981 | <0.001 | ||
| AFR | 0.923 | 0.879;0.970 | |||
| RCSL | 0.864 | 0.790;0.945 | <0.001 | ||
| AFR | 0.915 | 0.870;0.962 | |||
| Black-and-White Ruffed lemur Varecia variegata | Females (N = 269) Males (N = 239) | RCST | 0.950 | 0.929;0.970 | 0.20 |
| AFR | 0.926 | 0.888;0.966 | |||
| RCSW | 0.936 | 0.911;0.962 | 0.23 | ||
| AFR | 0.927 | 0.889;0.966 | |||
| RCSL | 0.888 | 0.846;0.933 | 0.14 | ||
| AFR | 0.925 | 0.887;0.965 | |||
| RCST | 0.975 | 0.955;0.995 | <0.001 | ||
| AFR | 0.919 | 0.882;0.956 | |||
| AFR | 0.936 | 0.902;0.970 | <0.001 | ||
| RCSL | 0.935 | 0.888;0.985 | 0.002 | ||
| AFR | 0.916 | 0.880;0.954 |
- Age at first reproduction (AFR) has a negative effect on mortality, except for females of RRL for which AFR was not retained in the selected model. Statistically significant effect of the random effect is indicated in bold.
For females of RRL, the selected model for the relationship between reproductive effort early in life and survival later in life included the reproductive effort metric, whatever it was (Table 2). Reproductive effort consistently had a positive effect on longevity (Table 2). For RCST, the mortality rate was reduced by a factor 1.05 (95%CI: [1.02;1.08], Table 2) indicating a decrease of 4.8% of mortality rate for each additional offspring produced (Figure 3a). RCSW had a stronger effect by decreasing by 5.5% the mortality rate for each additional offspring weaned (Table 2 and Figure S2a). RCSL had the strongest effect by decreasing the mortality rate by 12.4% for each additional litter produced (Table 2 and Figure S3a).
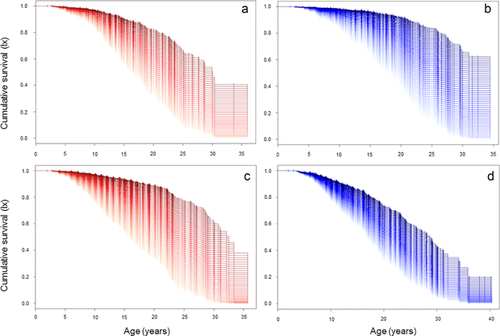
The selected model for the relationship between reproductive effort early in life and survival later in life in males of RRL consistently included the reproductive effort metric and AFR (Table 2). As for females, reproductive effort was positively associated with longevity (Table 2) with a decrease by 4.3% of mortality rate for each additional offspring produced (Figure 3b), a decrease by 6.0% for each additional offspring weaned (Table 2 and Figure S2b), and a decrease by 13.6% for each additional litter produced (Table 2 and Figure S3b). Moreover, reproducing for the first time later in life was positively related to longevity, with a decrease of mortality by 7.5–8.5% for each additional year prior to primiparity (Table 2).
For females in BWRL the selected model for the relationship between reproductive effort early in life and survival later in life consistently included the reproductive effort metric and the AFR (Table 2). In addition, for each reproductive metric, an additional reproductive effort unit led to decrease mortality by 5.0% (RCST), 6.4% (RCSW), and 11.2% (RCSL) (Table 2, Figures 3c, S2c, and S3c). Moreover, AFR was positively related to longevity with a decrease of mortality by 7.4–7.5% for each additional year prior to maturity (Table 2).
For males of BWRL, the selected model for the relationship between reproductive effort early in life and survival later in life included the reproductive effort (only when the metrics RCST or RCSL were used) and AFR (Table 2). Reproductive effort was positively associated with the longevity of male BWRL with a decrease of mortality by 2.5% for each additional offspring produced (Table 2 and Figure 3d) and a decrease by 6.5% for each additional litter (Table 2 and Figure S3d). However, the cumulative number of offspring successfully weaned did not have detectable influence on survival later in life (Table 2). Finally, AFR was positively related to longevity of male BWRL with a decrease of mortality by 6.5–8.2% for each additional year prior to sexual maturity (Table 2).
Finally, when individuals without any reproductive event during their lifetime were included in the analyses the direction of the effects remained the same but with a lower strength of the positive relationship between reproductive effort and longevity (statistically significant only in females, Table S2 and Figure S1).
4 DISCUSSION
Our work highlights that actuarial senescence does occur in captive populations of Varecia, in line with empirical evidence rapidly accumulating across vertebrates (Bouwhuis & Vedder, 2017; Bronikowski et al., 2011; Gaillard & Bonenfant, 2008; Gaillard, Garratt, & Lemaître, 2017; Jones et al., 2014; Larson et al., 2016; Nussey et al., 2013; Ricklefs, 2008). We found weak between-species differences in survival and actuarial senescence. Red ruffed lemurs tended to have lower baseline mortality and to senesce slightly faster than black and white ruffed lemurs, which led both species to have similar longevities (28–29 years). Overall, we did not detect any between-sex difference in actuarial senescence, but females tended to have lower baseline mortality and to senesce faster than males, leading to similar longevity in both sexes. Moreover, our study failed to detect any cost of reproductive effort in terms of late-life survival in captive populations of ruffed lemurs. On the contrary, a positive relationship occurred between reproductive effort early in life and longevity.
The slight difference of actuarial senescence between RRL and BWRL is not surprising because they are closely related species that share most of their ecological and physiological traits and are managed in similar ways in captivity. In the four datasets, the onset of senescence occurred 2–7 years after the age at sexual maturity, and around the mean AFR of the populations. As both species of ruffed lemurs are cooperative breeders (Baden et al., 2013; Tecot, Baden, Romine, & Kamilar, 2012; Vasey, 2007), we expected an onset of senescence much later than the AFR. The presence of helpers should lead to a decreased reproductive effort (Crick, 1992), which allows the allocation of the energy saved by parents to somatic maintenance and then potentially delays senescence (Bourke, 2007). This delayed effect of sociality on actuarial senescence has been recently reported in Alpine marmot (Marmota marmota, Berger et al., 2016), a cooperative breeder, with an onset of senescence occurring 3–5 years later than the AFR. It is important to keep in mind that the mean age at first reproduction has been obtained from captive individuals and it is likely that this late mean AFR reflects management rather than individual decisions. However, in the wild, females only reproduce for the first time during their 3rd year of life and no males have been observed mating before 5 years of age (Morland, 1991b), despite reaching sexual maturity between 18 and 20 months of age (Foerg, 1982). Hence, the onset of actuarial senescence was delayed in both Varecia species compared to their age at sexual maturity, but maybe not compared to their age at first offspring birth, measured in wild conditions.
Our results do not highlight between-sex differences in longevity and actuarial senescence in red ruffed lemurs, but females of both ruffed lemur species had lower baseline mortality and tended to senesce faster than males, leading to similar longevities in both sexes. The sex-difference was statistically significant in BWRL, but we can wonder whether this between-species difference in the amount of sex differences in survival and actuarial senescence involves a real physiological difference between species or results from the higher number of individuals available for BWRL, which allowed getting more precise estimates. A shorter longevity, an earlier age at the onset of senescence and a stronger rate of actuarial senescence are often observed in males compared to females in species with a polygynous mating system (Bronikowski et al., 2011; Clutton-Brock & Isvaran, 2007), whereas between-sex differences are absent or even reversed in monogamous species (Larson et al., 2016). In polygynandrous species, predictions in terms of between-sex differences in longevity and actuarial senescence are more complex, although in ruminants, sex-differences are more pronounced in promiscuous than in monogamous species (Tidière et al., 2015). For example, in the greater kudu (Tragelaphus strepsiceros), a promiscuous species, females start to senesce 1 year later, and at a slower rate than males, resulting in a longer (by 2 years) longevity (Tidière et al., 2015). However, in captive and wild populations of mouse lemurs (Microcebus spp.), a polygynandrous primate, Zohdy et al. (2014) failed to reveal any between-sex differences in survival, despite the high male-male competition for gaining access to mating opportunities. Moreover, no difference of actuarial senescence was observed in a population of northern muriquis in the wild, where both sexes mate with multiple partners without intra-sexual competition during the mating period (Bronikowski et al., 2011). In ruffed lemurs shared parental care between sexes and different members of the communities, along with both a high pre- and post-natal allocation to reproduction by females and an absence of competition between males during mating, could account for the weak between-sex differences of actuarial senescence and longevity we reported.
Contrary to what expected, our results revealed a positive effect of cumulative reproductive effort on late-life survival, and this effect was more pronounced in females than in males of both species. It is surprising that the positive effect of reproductive effort on longevity was greatest in females because the pre- and post-natal allocation to reproduction through embryo growth, parturition, and lactation is higher in females than in males. Indeed, pre-natal maternal care is greater in female Varecia than in any other primates (Young et al., 1990). The absence of a cost of reproductive effort in terms of survival and actuarial senescence could be accounted for by the high-quality environment provided by zoos. In captivity, individuals are protected from any form of competition for resources, because food and water are provided daily, and the risk of predation is mostly removed. Then, in such protected environments, we could expect that physiological costs associated with reproductive effort do not divert sufficient resources away from somatic maintenance to move individuals closer to a threshold for earlier age at death and faster senescence. These results are consistent with those reported by Ricklefs and Cadena (2007) in zoo populations of mammals and birds, or with those reported by Kengeri, Maras, Suckow, Chiang, and Waters (2013) on the North American Rottweiler dog meta-population.
The positive relationship between reproductive effort early in life and survival late in life is not as surprising as what one might think at first sight. Indeed, recent studies revealed that this pattern could be more widespread than expected. For instance, using longitudinal data on adult female mountain goats (Oreamnos americanus), Panagakis, Hamel, and Côté (2017) failed to find any negative relationship between allocation to reproduction in early life and rates of reproductive senescence and highlighted that a late AFR and a high early reproductive success both influenced positively the late-life reproductive performance. Twenty years ago, Bérubé, Festa-Bianchet, and Jorgenson (1999) already reported a slight positive relationship between early reproductive success and longevity in a population of bighorn sheep (Ovis canadensis). Moreover, the expected trade-off between reproduction and life span has also been challenged in invertebrates such as ant queens for which a strong enforced increase in reproductive effort did not reduce life span (Schrempf, Giehr, Röhrl, Steigleder, & Heinze, 2017).
The positive relationship between early-life allocation to reproduction and age at death we report in the present work supports the hypothesis that differences in individual quality (sensu, Wilson & Nussey, 2010) drive the observed differences in survival patterns. High quality individuals should perform better than low quality individuals in all biological functions, meaning that they acquire more energy and are enable both to produce more offspring and to live longer without suffering from any trade-off between early- and late-life performance. Trade-offs have been detected in several case studies of vertebrate populations, especially when differences in individual quality were accounted for (Lemaître et al., 2015). In a high-quality environment, the trade-off between early-life reproductive effort and late-life performance is expected to be minimal. In human populations, many studies highlighted that the intensity of the trade-off between reproduction and late-life survival or actuarial senescence decreases with increasing economic and social status (Doblhammer & Oeppen, 2003; Dribe, 2004; Korpelainen, 2000; Lycett, Dunbar, & Voland, 2000; Penn & Smith, 2007). Likewise, favorable zoo conditions may reduce the intensity of the trade-off between reproduction and survival, leading to an absence of detectable negative effects of early-life reproductive effort on the rate of actuarial senescence, and leaving individual quality to shape a positive correlation with age at death. In our study, we accounted for the natal environment conditions (both year and zoo at birth) to correct partly for measured individual heterogeneity, but we did not account for unmeasured individual heterogeneity. Although it would be interesting to include both measured and unmeasured individual heterogeneity to test whether a life-history trade-off between early-life reproduction and late-life survival still occurs, this is unlikely to change the patterns we report because lemurs live on the slow line of life histories and are thus expected to display very limited amount of individual heterogeneity in survival (Péron et al., 2016).
Studbook data revealed that maximum longevity of captive ruffed lemurs was higher than previously reported. Indeed, in the AnAge database (Tacutu et al., 2012), the maximum longevity of BWRL was recorded for a female that lived 37 years in captivity. Our study demonstrates that the maximum longevity is longer, with a male living up to 40 years. Additionally, there is no information about maximum longevity recorded for RRL in the AnAge database. We recorded a female living 35 years in captivity. Thus, ruffed lemurs seem to benefit a lot from captive environments. Ruffed lemurs have an important role in the Madagascar forest ecosystem: they play a major role in seed dispersal through their frugivorous diet (Moses & Semple, 2011). Conservation of ruffed lemurs is therefore crucial as they can be seen as keystone species.
ACKNOWLEDGMENTS
M.T. is funded by the French Ministry of Education and Research. We thank P. Dupont and A. Siberchicot for their useful advices. We are thankful to Samuel Pavard for his insightful comments that strongly improved the paper.
AUTHORS’ CONTRIBUTIONS
J.M.G., J.F.L., G.D., and M.T. designed the study; M.W., G.D., and M.T. collected the data; M.T., J.M.G., and J.F.L. analyzed the data; M.T. wrote the first draft of the manuscript that then received input from all other authors. Data used in this study were obtained from the published International Studbook for Ruffed Lemurs (2014, 2016).




