Seminal coagulation and sperm quality in different social contexts in captive tufted capuchin monkeys (Sapajus apella)
Abstract
In the present study, we aimed to assess the influence of different social contexts on the seminal coagulation and sperm quality in captive tufted capuchin monkeys. For this, males were housed either individually, in mixed-sex groups (with females), or in male-only groups. Monkeys were housed in cages and each cage type (i.e., individual or group cage) was placed in a different room. Forty-one males were subjected to semen collection by rectal electroejaculation. The degree of seminal coagulation was determined on a scale of I–IV. Seminal volume, sperm concentration, sperm motility, vigor, and plasma membrane integrity were evaluated for all ejaculate samples. All ejaculates collected showed degrees of coagulation between II and IV, where the majority presented coagulation degree IV, when collected from animals housed in groups. No statistical differences among percentages of coagula degree when samples were collected from males housed individually. Animals housed in group cages (male-only groups and mixed-sex groups) showed a significantly higher percentage of ejaculates at degree IV than males housed individually. Seminal volume was not affected by the coagula degree but by the housing system, where animals housed individually showed the highest volume (543 μl) when compared with those animals from male (273 μl) and mixed-sex (318 μl) groups. No differences were observed in semen volume when comparing male-only groups with mixed-sex groups. Sperm motility was affected by both housing system and coagula degree. Samples with coagula degree IV from animals housed individually showed the highest (72%) sperm motility percentages. Sperm plasma membrane integrity was lower when samples were presenting coagula degree II + III and collected from male- (17%) or mixed-sex (23%) groups. However, this housing system effect was not observed when sperm was obtained from coagula degree IV semen. Sperm vigor was neither affect by housing system or coagula degree.
1 INTRODUCTION
Seminal coagulation has been observed in several species, including humans (Dixson, 1998). Seminal coagulum is frequently found in the ejaculated semen of species where females mate with multiple partners (Dixson & Anderson, 2002). In primates, seminal consistency is species–specific, and is usually classified using a progressive four-point scale, where degree I represents fluid semen and degree IV represents a compact coagulum, the so-called copulatory plug (Dixson & Anderson, 2002). It was previously believed that copulatory plugs were absent of sperm and that their main function was to act as a physical barrier to future insemination by subsequently-mating males (chastity belt) (Kingan, Tata, & Rand, 2003; Voss, 1979). However, the presence of sperm in the seminal coagula of primates is still a matter for robust debate (Granados et al., 2014; Hernández-López, Cerda-Molina, Páez-Ponce, & Mondragón-Ceballos, 2008). The presence of sperm in the seminal coagula of some primate species living in multimale–multifemale social organizations (Dixson & Anderson, 2002), such as squirrel monkeys (Saimiri sciureus, Linnaeus, 1758) has not yet been reported (Bennet, 1967), leading to the conclusion that this coagulum has no fertilization properties. On the contrary, in other species with similar mating systems, such as spider monkeys (Ateles geoffroyi, Kuhl, 1820) (Hernández-López et al., 2008), tufted capuchin monkeys (Sapajus apella, Linnaeus, 1758) (Leão et al., 2015; Oliveira et al., 2011), and squirrel monkeys (Oliveira, Leão, Almeida, Santos, & Domingues, 2015; Oliveira et al., 2016a,2016b), seminal coagulum possesses high sperm concentrations. In fact, these coagulated fractions have been shown to possess higher sperm concentrations than the non-coagulated fractions of the ejaculate (Oliveira et al., 2011).
Sperm quality evaluation of seminal coagulum can clarify the evolutionary role of coagula in reproduction (Dorus, Evans, Wyckoff, Choi, & Lahn, 2004). If the presence of seminal coagulum varies among species in relation to their mating systems, the social context (i.e., living individually versus in a group) may also influence the variation of the ejaculate coagulation and the sperm concentration in the coagula. To date there is no available data relating the social context in which individual primates live to the consistency of their semen and their sperm quality. Animals housed in different social contexts may show significant variation in these semen qualities. Furthermore, if traditional studies on the reproductive physiology of Old World monkeys comparing their testicle sizes at the inter- and intra-specific differences of sperm competition (Bercovitch & Rodriguez, 1993; Dixson & Anderson, 2004; Harcourt, Purvis, & Liles, 1995; Jensen-Seaman & Li, 2003) generate insights into the evolution of male reproductive strategies in different social systems (Thomsen, 2014), then social context analyzed in this study may also influence some aspects of the semen.
In general, the S. apella alpha male copulates with different females, while high ranking females (not necessarily the alpha female) may copulate with different males, including dominant and subordinate males (Carosi, Linn, & Visalberghi, 2005; Janson, 1984; Phillips, Bernstein, Dettmer, Devermann, & Powers, 1994). However, not all adult males and females are able to monopolize one or more partners, and may go for long periods without engaging in any copulation at all, in nature and also in captivity (Wirz & Riviello, 2008). Low rates of aggressive behavior have been recorded among adult males of S. apella (Carosi et al., 2005; Matthews, 2012), along with low engagement and low interest in copulation. An important exception is observed in those events associated with the solicitation of proceptive females (Phillips et al., 1994) just before these females become fertile (Carosi et al., 2005), over a period of a few days, animals copulate very frequently and males outcompete each other to access the females.
Primates have become increasingly important for reproductive studies, mainly because of their high fertility in captivity (Fragaszy & Adams-Curtis, 1998; Wirz & Riviello, 2008). Semen collected from tufted capuchin monkeys presents a consistent coagulation (Nagle & Denari, 1983; Oliveira et al., 2011), which forms soon after ejaculation, and live sperm cells can be found inside the coagulum for many hours afterwards (Oliveira et al., 2011). This makes S. apella a very good model in which to study the evolutionary role of seminal coagulation among primates.
With the aim of assessing the effect of social context on seminal coagulation and sperm quality, we performed a series of analyses on semen collected from captive S. apella housed individually or in male-only or mixed-sex groups over a period of 5 consecutive years. We hypothesized that (i) animals housed in male-only or mixed-sex groups present more consistent semen coagula than those housed individually; (ii) sperm concentration in the coagula of animals housed in groups is more elevated; and (iii) sperm quality is improved in males living in a group as a form of post-copulatory sexual selection.
2 METHODS
All experimental protocols applied in the present study were approved by the Ethical Committee in Animal Research (n°013/2009/CEPAN/IEC/SVS/MS; 71/2013/CEPAE/UFPA) and by the Brazilian System of Authorization and Information in Biodiversity (SISBIO/ICMBio/MMA 34009-3; 41854-1). This research adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non-human Primates.
2.1 Study species
In the wild, tufted capuchins (S. apella) live in multimale–multifemale groups and polygamy is their usual mating system (Izar et al., 2012). Forty-one healthy, sexually mature S. apella males, approximately 15 years of age, had their semen collected from 2010 to 2014. The studied males were caught in the wild, therefore, their age was estimated based on dentition by considering tooth eruption, intra-osseous tooth formation and tooth wear (Smith, Crummett, & Brandt, 1994; Swindler, 2002). The exact age at which this species stops producing sperm is unknown, but it is well-known that, when maintained in captivity, S. apella can live for more than 50 years (Janson, 2006; Nagle & Denari, 1983).
2.2 Animal selection and housing conditions
The experimental animals were caught in the Brazilian Amazon rainforest and kept in two types of indoor cages (individual or in male-only or mixed-sex groups) at the National Primate Center (CENP), Ananindeua, Pará State, Brazil (1°2′57”S, 48°22′52”W). Individual cages had dimensions of 0.9 × 0.8 × 0.8 m (length, width, and height, respectively) and group cages had dimensions of 3.85 × 1.20 × 2.40 m. Individual cages were maintained in a different room from the group cages. The group cages held groups of four or five animals, where one male lived with three females in the mixed-sex groups, and all cages were placed into a same room. We evaluated 21 males housed individually, 8 males in male-only groups and 12 males in mixed-sex groups. The local climate is tropical humid, with an average annual temperature of 28°C, and the animals were kept under the natural photoperiod, that is, 12 hr of light and 12 hr of dark. Their diet consisted of fresh fruits, vegetables, commercial primate chow, and drinking water, available ad libitum.
2.3 Semen collection
Semen was collected under standard conditions (Oliveira et al., 2011), only in the morning and always before the feeding routine. Animals were first anesthetized using ketamine hydrochloride (15 mg/kg; IM; König S.A., Avellaneda, Argentina) and xylazine hydrochloride (1 mg/kg; IM; König S.A.). Animals were stimulated with a modified rectal electroejaculation (EEJ) procedure. An electroejaculator (AutoJac®; Neovet, Uberaba, Minas Gerais, Brazil) rectal probe (0.9 cm diameter and 15 cm long), previously lubricated, was introduced in the rectum and positioned near the prostate (∼5 cm deep) and electrical stimuli were delivered. A stimulation session consisted of three series of 35 crescent electrical stimuli (10–100 mA; 1–10 V) with an interval of 30 s between each series. If a male was unable to ejaculate after the electroejaculation session, no further attempts were made to collect semen. The intervals between semen collections (successful or unsuccessful attempts) were at least 30 days. Due to this, evaluated semen was obtained from ten animals in individual cages, five animals in male-groups and eleven animals in mixed-sex groups cages (Supplementary Table S1).
A veterinarian continually monitored the animals during procedures and after recovering from anesthesia. After recovery from anesthesia the animals were transported back to their original cages and were monitored until total recovery.
2.4 Semen evaluation
Seminal volume and consistency were immediately evaluated after ejaculation, with the ratings of semen coagulation made by the same person. Seminal volume was evaluated in a graduated micro centrifuge tube. Coagulum volume was calculated as the total seminal volume minus the liquid volume. The consistency of seminal coagulation was classified according to a modified subjective scale described by Dixson and Anderson (2002), where: degree I = fluid semen; degree II = gelatinous semen; degree III = semi-solid semen; degree IV = solid coagulum, which is a compact coagulum also denoted as a copulatory plug. Spermatic motility, vigor, sperm concentration and plasma membrane integrity were immediately evaluated after partial mechanical fragmentation. For this, the extender ACP-118™ (powdered coconut water; ACP Biotecnologia, Fortaleza, Ceará, Brazil), which is commonly used as a seminal extender for Neotropical primates (Leão et al., 2015; Lima et al., 2013; Oliveira et al., 2015, 2016a,2016b) and other mammals (Cardoso et al., 2007; Silva et al., 2015, 2012; Uchoa et al., 2012) was added to each seminal coagulum (1:1) and maintained in a water bath (36°C) to allow coagulum liquefaction. Each coagulum was periodically (every 10 min) gently mixed with the help of a pipette tip in order to improve sample homogeneity (Oliveira et al., 2015). Spermatic motility, vigor and sperm concentration were assessed according to Oliveira et al. (2015). A smear preparation was used to determine plasma membrane integrity, with 5 μl of 1% eosin and 5 μl of 1% nigrosine added to 5 μl of semen on a pre-warmed (36°C) glass slide. A total of 100 cells were counted per slide. All evaluations were performed under a light microscope (Nikon Eclipse E400®; Nikon Instruments Europe B.V.; Amsterdam, the Netherlands) at a magnification of 100×.
2.5 Statistical analysis
Statistical analysis was conducted with the GenStat statistical software (GenStat for Windows 14th Edition, VSN International, Hemel, Hempstead, UK, Available online at: https://www.vsni.co.uk/downloads/genstat/). Each male was an experimental unit. Data were not normally distributed. Therefore, differences in seminal coagulation degree (I, II, III, and IV) among the three social contexts, that is, animals housed individually, in male-only and mixed-sex groups, were compared with generalized linear mixed model (GLMM), and are presented as range and mean ± SE. The effects of coagula degree (II + III and IV) and social context (animals housed individually, in male-only and mixed-sex groups) on the semen volume, sperm motility, sperm vigor, and sperm plasmatic membrane integrity were also evaluated with GLMM, where: response parameters were semen volume, sperm motility, sperm vigor, and plasma membrane integrity and the fixed model was coagula degree and housing system (social context), and are presented as mean ± SE. Before statistical analysis, data were transformed to log. Tests were two-tailed, with alpha set at 0.01.
3 RESULTS
From a total of 189 electroejaculation attempts, semen was obtained in 109 cases (58%), from which 61 seminal samples (56%) had a sufficient volume for analysis. We observed a high frequency of masturbation in males enclosed with females during the semen collection period. However, it was not possible to quantify all the masturbation events every day. From the males in mixed-sex groups, only dominant males were able to copulate several times with females. We observed a lower frequency of masturbation in the individual and male-only groups. The consistencies of the collected ejaculates ranged from degree II to degree IV (Figure 1A–C). The ejaculates with a consistency at degree IV tended to present a shape similar to the contour of the male urethra. This was not observed in degree II and III ejaculates. None of the parameters was affected by the year of collection.
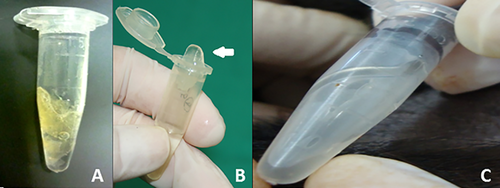
None of the tested groups presented ejaculates classified as degree I. The mean percentage of ejaculates presenting coagulation degrees II, III, and IV in animals housed individually were 50% (range of 20–67%), 11% (range of 8–25%), and 39% (range of 13–80%) respectively, and no significant differences were observed between these groups (p > 0.999). In contrast, monkeys from the male-only group presented significantly higher (p < 0.0001) percentages of ejaculates at degree IV (73%; range of 60–100%) than degrees II (none) or III (27%; range of 0–40%). Similar results were obtained in the mixed–sex group where the highest (p < 0.0001) percentages of ejaculates at degree IV (72%; range of 50–100%) were observed when compared to degrees II (17%; range of 0–40%) and III (11%; range of 0–33%) (Table 1). Ejaculates were collected over a five years’ period and no effect of year on coagula degree was observed.
| Housing (social context) | |||
|---|---|---|---|
| Coagula degree | Individual cage mean % (range) | Male-only cage mean % (range) | Mixed-sex cage mean % (range) |
| II (%) | 50a (20–67) | 0a (0–0) | 17a (0–40) |
| III (%) | 11a (8–25) | 27a (0–40) | 11a (0–33) |
| IV (%) | 39a (13–80) | 73b (60–100) | 72b (50–100) |
| SE | ±8.3 | ±9.9 | ±9.9 |
| P source of variation | |||
| Degree effect | >0.9999 | <0.0001 | <0.0001 |
| Housing effect | >0.9999 | >0.9999 | >0.9999 |
| Degree × housing | 0.1024 | 0.0367 | 0.0428 |
- None of the ejaculates presented coagula degree I.
- No statistical differences were observed among social contexts within the same coagula degree.
- a,bDifferent uppercase letters indicate statistical difference among coagula degree within the same social context.
There was no significant difference between sperm concentrations of males living in different social contexts (p > 0.999). The average (mean ± SE) sperm concentration of animals living in individual cages was 608 ± 98 × 106 sperm/ml, while this value for all animals in group cages was 416 ± 162 × 106 sperm/ml (no significant differences were detected between male-only and mixed-sex groups).
In Table 2, we present the average values of seminal characteristics (volume, sperm motility, vigor, and plasma membrane integrity) as determined in animals housed individually, in male-only groups or in mixed-sex groups. Due to the absence of coagula at degree I, as well as an insufficient number of animals presenting semen with coagula at degrees II and III to allow for statistical analysis, data are presented for semen collected at degree IV only, or as a pool of ejaculates with coagula degree II + III. Semen volume was significantly greater (p < 0.01) in males housed individually (543 μl) when compared to those in male-only (273 μl) or mixed-sex (318 μl) groups, independently on the coagula degree (p = 0.210). Sperm motility was affected by both coagula degree and social context (p < 0.001). Motility of sperm harvested from coagula degree IV was significantly higher (p < 0.001) than of sperm recovered from coagula degree II–III. Furthermore, sperm motility was also significantly higher (p < 0.001) in semen collected from males housed individually when compared to those housed in male groups or mixed-sex groups. Sperm motility from males housed in male-groups was not different from that recorded in males from mixed-sex groups (p > 0.01). Sperm plasma membrane integrity was influenced by the social context depending on the coagula degree, where no effect was observed for membrane integrity when sperm from only coagula degree IV were analyzed, while from semen with coagula degree II + III, sperm from males housed individually presented a significantly higher percentage of plasma membrane integrity when compared to the male-only and mixed-sex groups (p < 0.001). Also, coagula degree IV semen from animals caged in groups (male only or mixed-sex) presented higher rates of membrane integrity when compared with coagula degree II + III also obtained from animals caged in groups (male only or mixed-sex). Sperm vigor was affected neither by the coagula degree (p = 0.493) or by the housing system (p = 0.103).
| Evaluated parameters | |||||
|---|---|---|---|---|---|
| Coagula degree | Housing (social context) | Seminal volume (µl) | Sperm motility (%) | Sperm vigor (0–5) | PMI (%) |
| II + III | Individual cage (N = 17 males) | 513a | 54a,A | 4 | 66a,A |
| Male-only cage (N = 4 males) | 233b | 15b,A | 2 | 17b,A | |
| Mixed-sex cage (N = 10 males) | 250b | 15b,A | 2 | 23b,A | |
| IV | Individual cage (N = 17 ales) | 573a | 72a,B | 4 | 54a,A |
| Male-only cage (N = 4 males) | 312b | 43b,B | 3 | 45a,B | |
| Mixed-sex cage (N = 10 males) | 386b | 36b,B | 2 | 31a,B | |
| SE | ±29 | ±5 | ±0.2 | ±5 | |
| Coagula degree | |||||
| II + III | 332 | 28a | 3 | 35a | |
| IV | 424 | 50b | 3 | 43b | |
| Housing | |||||
| Individual | 543a | 63a | 4 | 60 | |
| Male-only | 273b | 29b | 3 | 31 | |
| Mixed sex | 318b | 26b | 2 | 27 | |
| P source of variation | |||||
| Degree effect | 0.793 | <0.001 | 0.493 | <0.001 | |
| Housing effect | 0.005 | <0.001 | 0.103 | 0.290 | |
| Degree × housing | 0.210 | <0.001 | 0.886 | 0.007 | |
- Data are presented for those semen classified as degree IV or as a pooled sample of degrees II and III, independent of the coagula degree.
- PMI, Plasma membrane integrity.
- a,bDifferent lowercase letters indicate statistical difference among social context within the same coagula degree.
- A,BDifferent uppercase letters indicate statistical difference among coagula degree within the same social context.
4 DISCUSSION
In this study, we describe not only the occurrence of intraspecific variation in the properties of semen from S. apella, but we also demonstrate that this variation is related to the social context of the animals studied (i.e., when housed individually, in male-only or in mixed-sex groups). In our samples, animals housed in the male-only and mixed-sex groups presented more frequently the most solid coagulum state (degree IV), which was not observed in those housed individually, as hypothesized. These results may be due to the fact that animals living together increase solidification of their seminal coagula as a form of post-copulatory sexual selection, as previously described (Parga, 2003). Animals living in group cages can also benefit from more coagulated ejaculates since the seminal coagulum can keep the sperm cells alive for longer periods of time, releasing sperm gradually into the female reproductive tract (Suarez & Pacey, 2006). The consistency of ejaculated semen varies substantially among primates, most notably in the presence of a solid coagulum in promiscuous species (Jensen-Seaman & Li, 2003). Such a coagulum is formed mainly by proteins originating from the seminal vesicles (De Lamirande, 2007; Granados et al., 2014; Robert & Gagnon, 1999; Valtonen-André, Olsson, Nayudu, c Lundwall, 2005). Biochemical mechanism studies have found that two of the primary proteins present in seminal coagulum are seminogelin I (Sg I) and seminogelin II (Sg II) (Robert & Gagnon, 1999). In humans, semen coagula liquefy spontaneously within 5–20 min (Lilja, Oldbring, Rannevi, & Laurell, 1987; Robert & Gagnon, 1999), due to the secretion of a prostatic-specific antigen in the seminal plasma (Robert & Gagnon, 1999) that interacts with the Sg and fibronectin proteins (Lilja et al., 1987). In contrast, in other primates such as S. apella, one part of the semen is ejaculated already coagulated and no spontaneous liquefaction is observed ex vivo (Leão et al., 2015; Lima et al., 2013; Oliveira et al., 2011). When collected for assisted reproductive techniques, the highly viscous seminal portion is usually not used due to its difficult liquefaction (Valle et al., 2004), which requires mechanical fragmentation (Oliveira et al., 2011) or enzymatic digestion (Flores-Herrera et al., 2012; Granados et al., 2014). Therefore, for many years, this important portion of the ejaculate has been neglected.
Another function of the Sg protein is to act as a precursor of the seminal plasma motility inhibitor (SPM I) (Luterman, Iwamoto, & Gagnon, 1991), which inhibits sperm motility (De Lamirande, 2007; Robert & Gagnon, 1996). The SPM I blocks sperm motility by inhibiting the dynein ATPase, which is responsible for the functioning of microtubule motors from the axonema (De Lamirande, Bardin, & Gagnon, 1983; De Lamirande, 2007). This may explain why sperm motility is absent or low within the seminal coagulum soon after ejaculation and increases progressively during coagula liquefaction (De Lamirande, 2007). In the present study, sperm motility was the lowest when semen at degree IV was collected from male-only groups or mixed-sex groups probably due to a higher concentration of Sg in the semen (Robert & Gagnon, 1999).
Seminal coagulum from S. apella has a high sperm concentration, with a potential role in fertilization. Differently from our hypothesis, we did not observe a higher sperm concentration in semen from animals housed in groups (male-only or mixed-sex). Previously, Dixson and Anderson (2002) described seminal coagula molded to the contours of the female reproductive tract, because they assumed it would be efficient in blocking any subsequent fertilization attempt. Our results, however, showed that solid coagulum was usually shaped to the contours of the male urethra. Furthermore, the “chastity belt” hypothesis has been excluded in primates. In wild tufted capuchin monkeys, Lynch-Alfaro (2005) observed many coagulated pieces of semen being expelled from a female's vagina during rapid sequential mating. Hence, a second male's mount series is usually sufficient to remove prior ejaculate from the female vagina, as an evolutionary advantage to polygamous mating systems where repeated intromissions facilitate the expulsion of prior plugs (Lynch-Alfaro, 2005).
Alternative functions of the seminal coagulum have also been proposed, including the protection of spermatozoa against vaginal acid medium in primates (Dixson & Anderson, 2002) or the prevention of sperm leakage by retaining sperm inside the rat female tract (Matthews & Adler, 1978). However, the coagulum does not act as a barrier to subsequent males attempting to copulate with the same females (Dixson, 2012). Sequential copulation of a single female by many males of non-human primate species has been observed many times in the wild and captivity (Carosi et al., 2005; Lynch-Alfaro, 2005; Matthews, 2012; Phillips et al., 1994). Therefore, we propose that the most coagulated seminal portion (degree IV) should be called “solid coagulum” and not “copulatory plug.”
Seminal coagulum production has a high energy cost for primate males. Thomsen et al. (2006) reported that ejaculates are produced with substantial cost for Japanese macaque (Macaca fuscata, Linnaeus, 1758) males. From this study, the calculated relative energy for an ejaculation from Japanese macaque males ranged from 0.8 to 6% of their daily basal metabolic rate. In addition, Hernández-López et al. (2008) reported the reproductive strategies for males of Ateles geoffoyi, where the most seminal coagulum production coincided with the season of highest sexual activity in the females, in which their optimal reproductive function occurs.
Bush, Russel, Flowers, and Sorensen, (1975) reported that semen obtained by eletroejaculation, or removed from the reproductive tract of females, always contained a coagulated fraction, and that coagulation took place soon after ejaculation. In the present investigation, we found no liquid fraction in the ejaculates, regardless of their consistency, since all ejaculated volumes showed some degree of coagulation (degrees II, III, and IV).
Seminal parameters may be useful for identifying characteristics which can be considered reliable attributes of the animal's fertility status (Kholkute, Gopalkrishnan, & Puri, 2000) and individual differences in semen quality have been found in captive (Kuederling, Schneider, Sønksen, Nayudu, & Hodges, 2000) and wild (Thomsen, 2014) primates. Although some primatologists seem to assume that all males exhibit similar fertilizing potentials (Dubuc, Muniz, Heistermann, Engelhardt, & Widdig, 2011; Widdig et al., 2004), our results demonstrate that animals housed individually showed better semen quality (more volume), sperm motility and plasma membrane integrity rates than animals housed in groups. Therefore, our third hypothesis was not supported by the findings. This result suggests that social stress (e.g., competition for food and/or for partners), the higher levels of aggression that this context might represent, and the variation in the physiological conditions resulting from that stress, may affect sperm quality. In the present study, liquefaction of the seminal coagula was obtained by mechanical fragmentation, since spontaneous liquefaction is not observed in this species. The mechanical method has less effect on the integrity of the sperm membrane (Leão et al., 2015; Oliveira et al., 2015) than the addition of proteolytic enzymes (e.g., trypsin) (Flores-Herrera et al., 2012; Hernández-López, Cerezo-Parra, Cerda-Molina, Pérez-Bolaños, & Mondragóná1Ceballos, 2002).
In general, when male-only and mixed-sex groups were compared, the values of seminal volume, sperm motility, vigor, and membrane integrity were similar. It means stress having few importance in social context, represented as the “sex context,” in the development of post-copulatory strategies for competition among males. In most animal species, dominance rank is largely based on fighting abilities, and thus should provide a reproductive advantage, both through mechanisms of male–male competition and/or female mate choice for superior males (Clutton-Brock & McAuliffe, 2009). However, in primates, this general pattern deviates by showing an unusually high degree of variability in male reproductive skew (Dubuc et al., 2011). Carosi et al. (2005) pointed out that capuchin dominant males can wait for proceptive females to mate with many subordinate males before they finally copulate. As other authors argue (Janson, 1984; Lynch-Alfaro, 2005), apparently S. apella males do not usually produce semen in volumes high enough to inseminate a large number of proceptive females.
Other possible factors may also be considered as potential sources of the variation of ejaculate properties and sperm quality. Animals, when in groups, engage in sexual activity more frequently (Wirz & Riviello, 2008), and masturbatory activity can be more frequent in the presence of other primates (Zimmerman, Maude, & Moldawer, 1965). Although it was not possible to register all the events, in our study, high frequencies of masturbation were observed in males enclosed with females, while those males housed alone or just with males showed low frequencies of masturbation. The adaptive function of male masturbation is still poorly understood, despite its high prevalence in humans and non-human primates living in polygamous mating system (Dubuc, Coyne, & Maestripieri, 2013; Thomsen & Soltis, 2004) and in the wild (Thomsen, 2014). Masturbation could lead to lower ejaculate volume and reduced sperm number, but the best ejaculate quality is the next ejaculate, which is used for mating and may contain fewer but more motile sperm (Zimmerman et al., 1965). Furthermore, males can discard old sperm from ejaculate (Thomsen, Soltis, & Teltscher, 2003). Thus, any sexual activity leading to a continued or frequent discharge of ejaculate may influence some of these seminal properties (Thomsen et al., 2003). Consequently, from the point of view of captive capuchins management, it would be recommended that the animals are kept individually, at least for a few days before seminal collection, in order to harvest better quality sperm.
This is the first study describing the existence of more than one degree of seminal coagulation for captive tufted capuchin monkeys. We have demonstrated here that the social context in which animals live promotes changes in some seminal characteristics in this species, although the presence of females apparently is not relevant in this regard. The male-only and mixed-sex groups showed higher degrees of coagulation in their semen. In general, animals housed in individual cages showed larger seminal volume and sperm motility when compared with those housed in group cages. More studies are necessary to understand whether this characteristic is somehow an advantage. These findings provide new information on the seminal parameters of S. apella, and will support future reproductive studies on this, and on other Neotropical primate species.
ACKNOWLEDGMENTS
The authors thank “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for financial support (Portaria n° 76, de 14/04/10—Novo Regulamento—Demanda Social) and the “Centro Nacional de Primatas” for the technical support (Acordo de Cooperação Acadêmica e Técnico-Científica n°1/2014) during this research.




